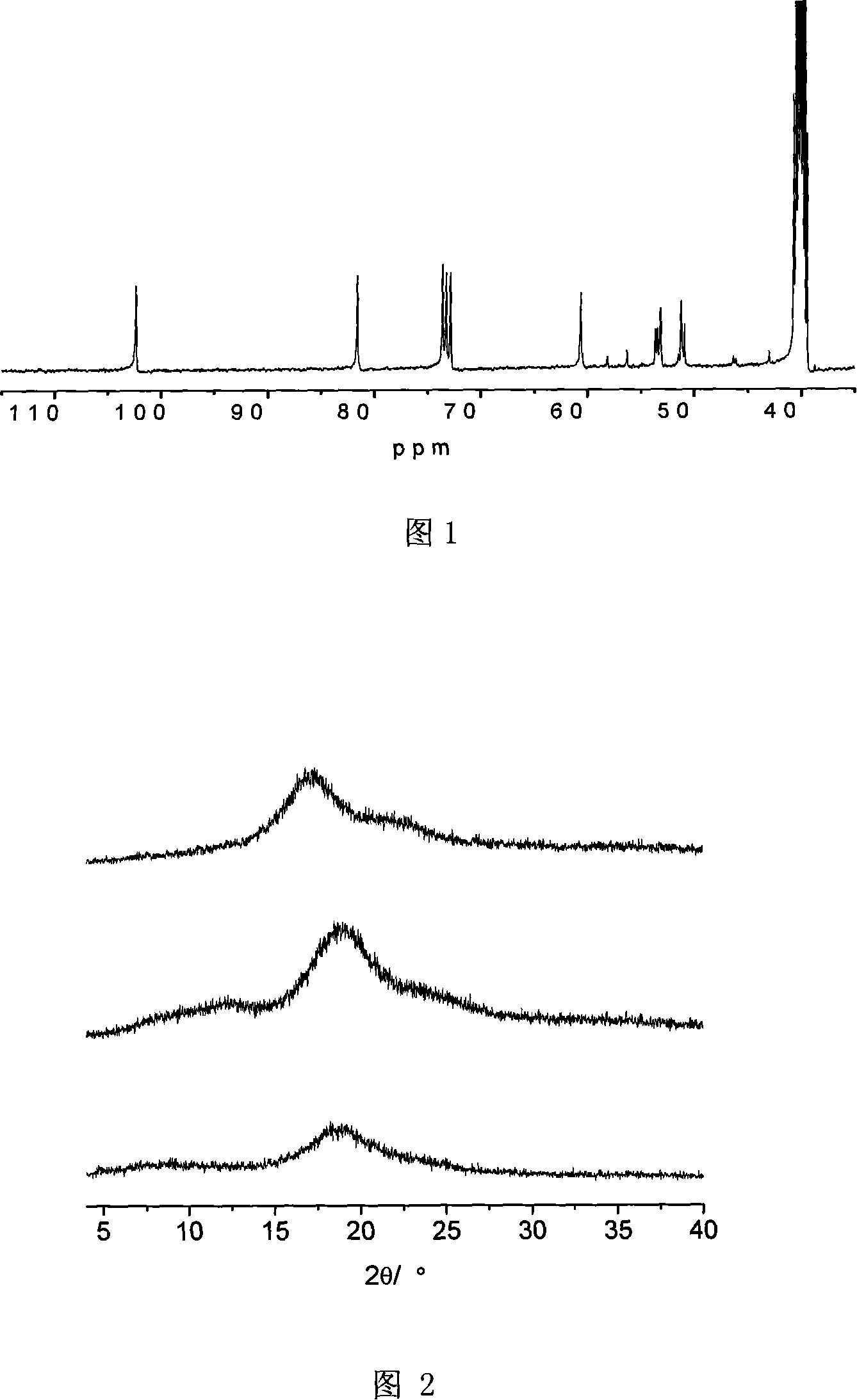
Method for preparing hyper branched polyrotaxane based on cyclodextrin
A technology of cyclodextrin and polyrotaxane, which is applied in the field of preparation of hyperbranched polyrotaxane, and achieves the effects of simple preparation, high yield and easy structure adjustment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Step a: Add 20 milliliters of deionized water to a 100 milliliter reaction flask, then add 1.297 grams of gamma-cyclodextrin to dissolve it, then add 0.1292 grams of 1-(2-aminoethyl) piperazine, at 70 degrees Celsius Stirred under conditions for 5 hours, cooled naturally to obtain a light yellow solution.
[0025] Step b: Add 0.1182 g of divinyl sulfone to the small molecule solution obtained above, seal it with an inversion plug, blow nitrogen for 10-15 minutes to remove the air, seal it well, stir at room temperature for 3-5 minutes, and then heat to 40 Stirring was continued for 120 hours at °C.
[0026] Step c: Add 20 ml of petroleum ether into the reaction bottle after the reaction, and continue stirring at room temperature for about 3-5 hours to obtain a cloudy solution. The precipitate was removed by centrifugation to obtain a clear biphasic solution. The upper layer is excess petroleum ether, and the lower layer is light yellow aqueous solution of hyperbranche...
Embodiment 2
[0028] Step a: Add 20 ml of deionized water to a 100 ml reaction flask, then add 0.0045 g of β-cyclodextrin to dissolve it, then add 0.1162 g of hexamethylenediamine, stir at 70 degrees Celsius for 5 hours, and cool naturally to obtain Pale yellow solution.
[0029] Step b: Add 0.1542 g of methylene bisacrylamide to the small molecule solution obtained above, seal it with an inversion plug, blow nitrogen for 10-15 minutes to remove the air, seal it well, stir at room temperature for 3-5 minutes, and then heat Stirring was continued for 120 hours at 40°C.
[0030] Step c: Add 20 ml of petroleum ether into the reaction bottle after the reaction, and continue stirring at room temperature for about 3-5 hours to obtain a cloudy solution. The precipitate was removed by centrifugation to obtain a clear biphasic solution. The upper layer is excess petroleum ether, and the lower layer is light yellow aqueous solution of hyperbranched polyrotaxane. The excess petroleum ether was remo...
Embodiment 3
[0032] Step a: Add 20 ml of deionized water to a 100 ml reaction flask, then add 0.4635 g of α-cyclodextrin, then add 0.1292 g of 1-(2-aminoethyl)piperazine, and stir at 70 degrees Celsius After 5 hours, cool naturally to obtain a light yellow solution.
[0033] Step b: Add 0.1542 g of methylene bisacrylamide to the small molecule solution obtained above, seal it with an inversion plug, blow nitrogen for 10-15 minutes to remove the air, seal it well, stir at room temperature for 3-5 minutes, and then heat Stirring was continued for 120 hours at 40°C.
[0034] Step c: Add 20 ml of petroleum ether into the reaction bottle after the reaction, and continue stirring at room temperature for about 3-5 hours to obtain a cloudy solution. The precipitate was removed by centrifugation to obtain a clear biphasic solution. The upper layer is excess petroleum ether, and the lower layer is light yellow aqueous solution of hyperbranched polyrotaxane. The excess petroleum ether was removed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com