Application of BRSK2 in producing antidiabetic medicine
An anti-diabetic and drug technology, applied in the field of biomedicine, can solve problems such as the effect of BRSK2 that has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
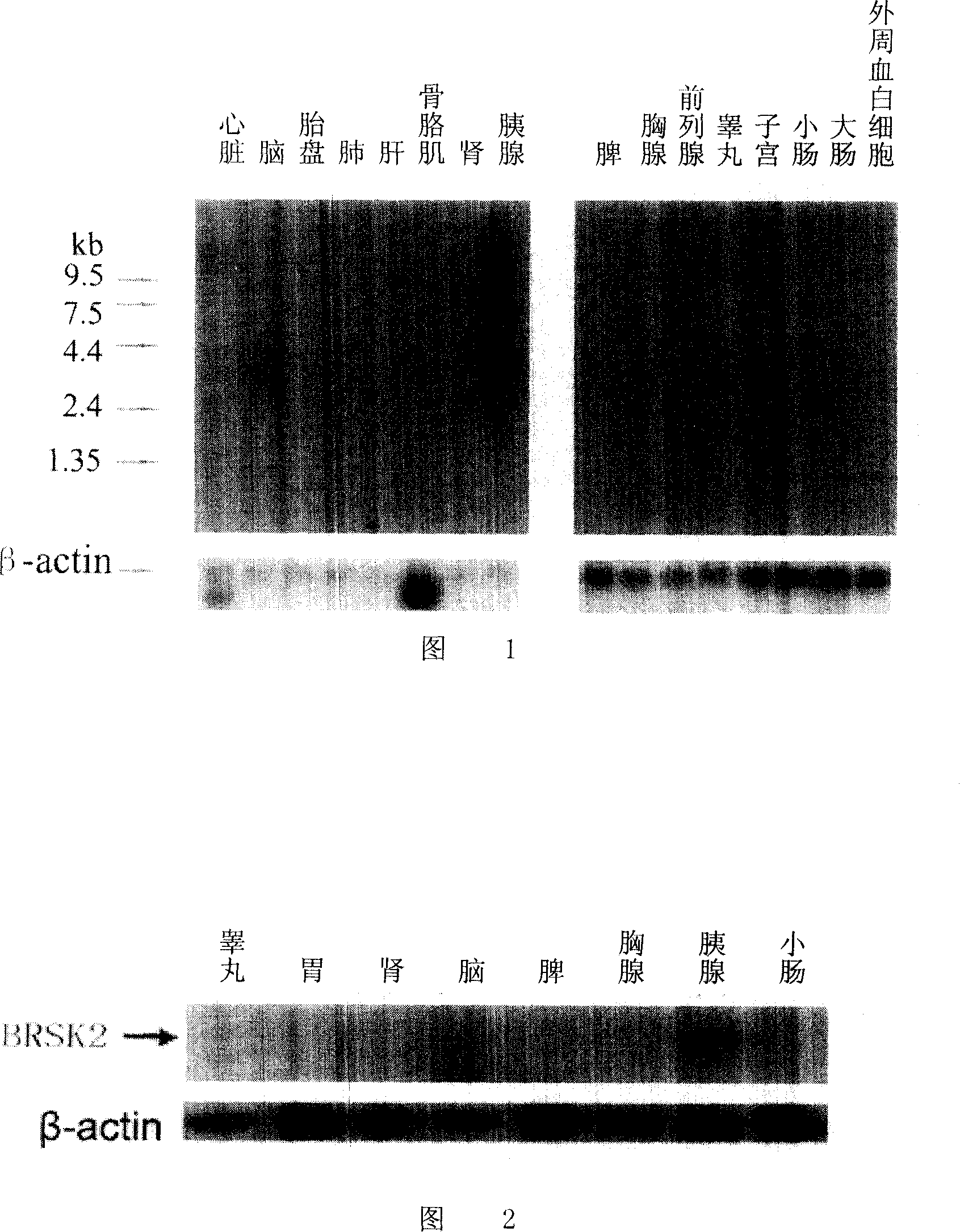
[0046] Example 1 The specific steps of Northern hybrid expression of BRSK2 in pancreas are:
[0047] 1.1 cDNA probe preparation and purification
[0048] (1) Using specific primers of BRSK2 gene to amplify the PCR product from the constructed and sequenced plasmid. After the PCR product was purified with the QIAquick PCR purification kit, the concentration of the purified DNA was measured with a GeneQuant II UV spectrophotometer.
[0049] (2) After the DNA template was heat-denatured at 100°C for 2 minutes, the random primer labeling kit was used to incorporate [α- 32 P] dCTP. After the labeling reaction, EDTA was added to a final concentration of 20mmol / L to stop the reaction, heat denatured at 100°C for 5min, and placed on ice.
[0050] (3) Purify the probe with MicroSpin G-25 purification column. Before and after purification, 1 μl of the sample was taken and diluted 100 times, and 3 μl of the diluted solution was spotted on Whatman DE81 filter paper, and the radiation ...
Embodiment 2
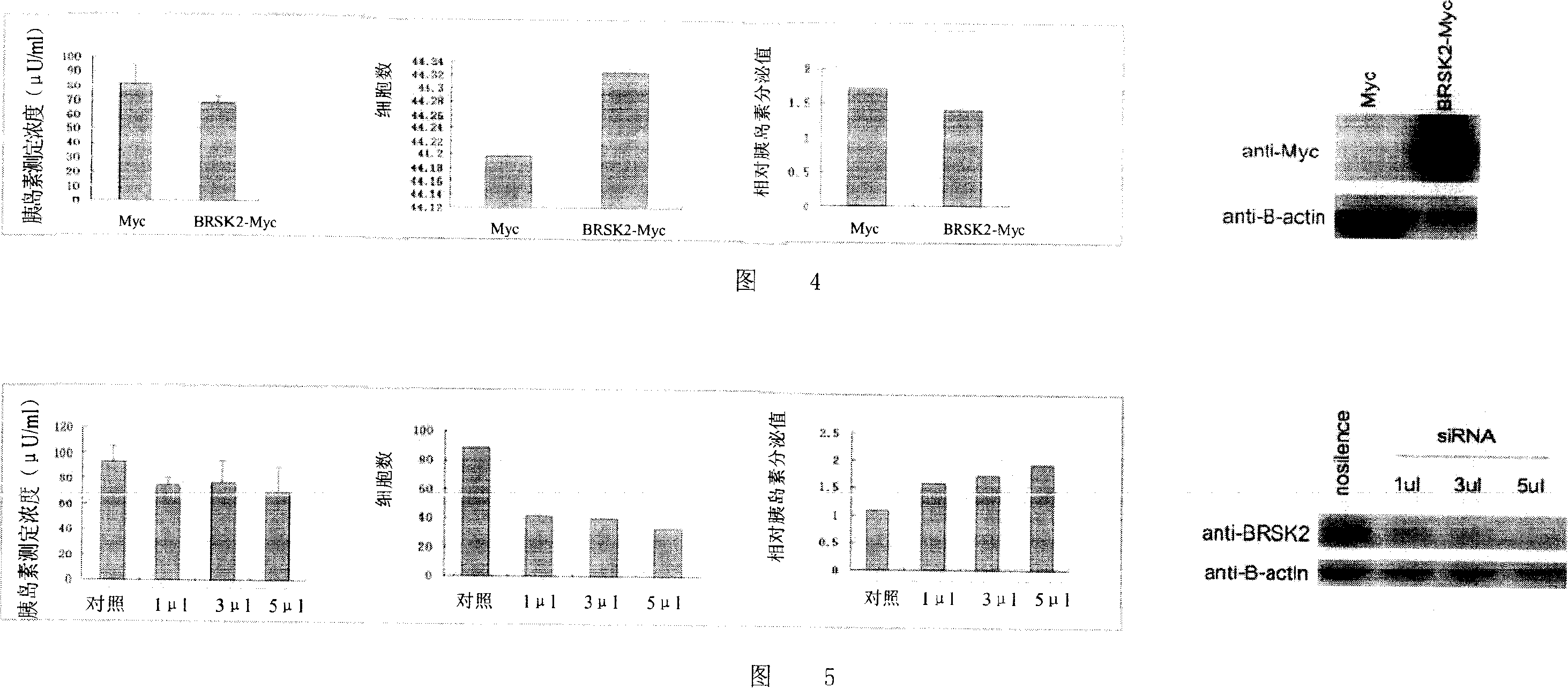
[0057] Embodiment 2 prepares antibody
[0058] BRSK2 protein is used to immunize animals to produce antibodies, the specific method is as follows. The recombinant molecules are separated by chromatography for further use. It can also be separated by SDS-PAGE gel electrophoresis, and the electrophoresis bands are excised from the gel and emulsified with an equal volume of complete Freund's adjuvant. Mice were injected intraperitoneally with 50-100 [mu]g / 0.2 ml emulsified protein. Fourteen days later, mice were boosted by intraperitoneal injection of the same antigen emulsified with incomplete Freund's adjuvant at a dose of 50-100 µg / 0.2 ml. Give booster immunizations at least three times every 14 days. The specific reactivity of the obtained antiserum was assessed by its ability to precipitate the human BRSK2 gene translation product in vitro. As a result, it was found that the antibody can specifically precipitate the protein of the present invention.
Embodiment 3
[0059] Example 3 BRSK2 Western hybridization experiment in pancreas
[0060] 3.1 Extraction of mouse tissue proteins
[0061] (1) All excised tissues were quickly frozen into liquid nitrogen tanks and stored at -80°C.
[0062] (2) Take 50-100 mg of tissue samples and add 400 μl of cell lysate (containing protease inhibitors).
[0063] (3) Grind the above-mentioned tissue sample with a grinder until it becomes a suspension.
[0064] (4) Add 100 μl of 5X protein loading buffer to 400 μl of the suspension, and boil at 100° C. for 10 minutes.
[0065] (5) Centrifuge at 12,000 rpm for 5 minutes at 4°C, and the supernatant can be used for sample loading.
[0066] 3.2 Western detection of endogenous expression of BRSK2 in various tissues of mice
[0067] (1) Prepare SDS-PAGE separating gel and stacking gel.
[0068] (2) Perfusion of the gel (the process is as follows)
[0069] Perfuse separation gel-→add absolute ethanol to remove air bubbles-→stand at room temperature and wait...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com