Andrographolide derivatives and application of the same in pharmacy
A technology of andrographolide, derivatives, applied in antipyretic, antibacterial, antitumor and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
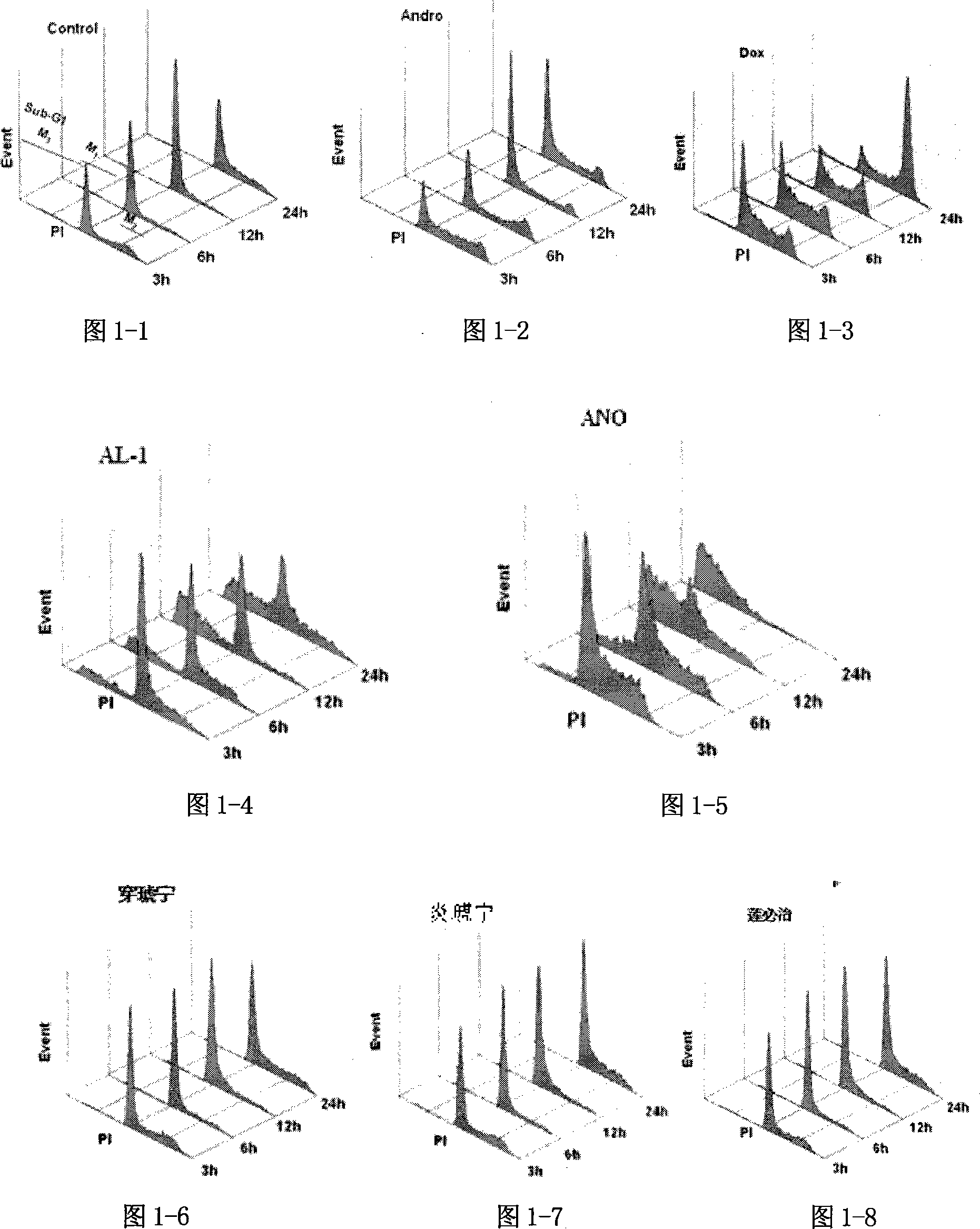
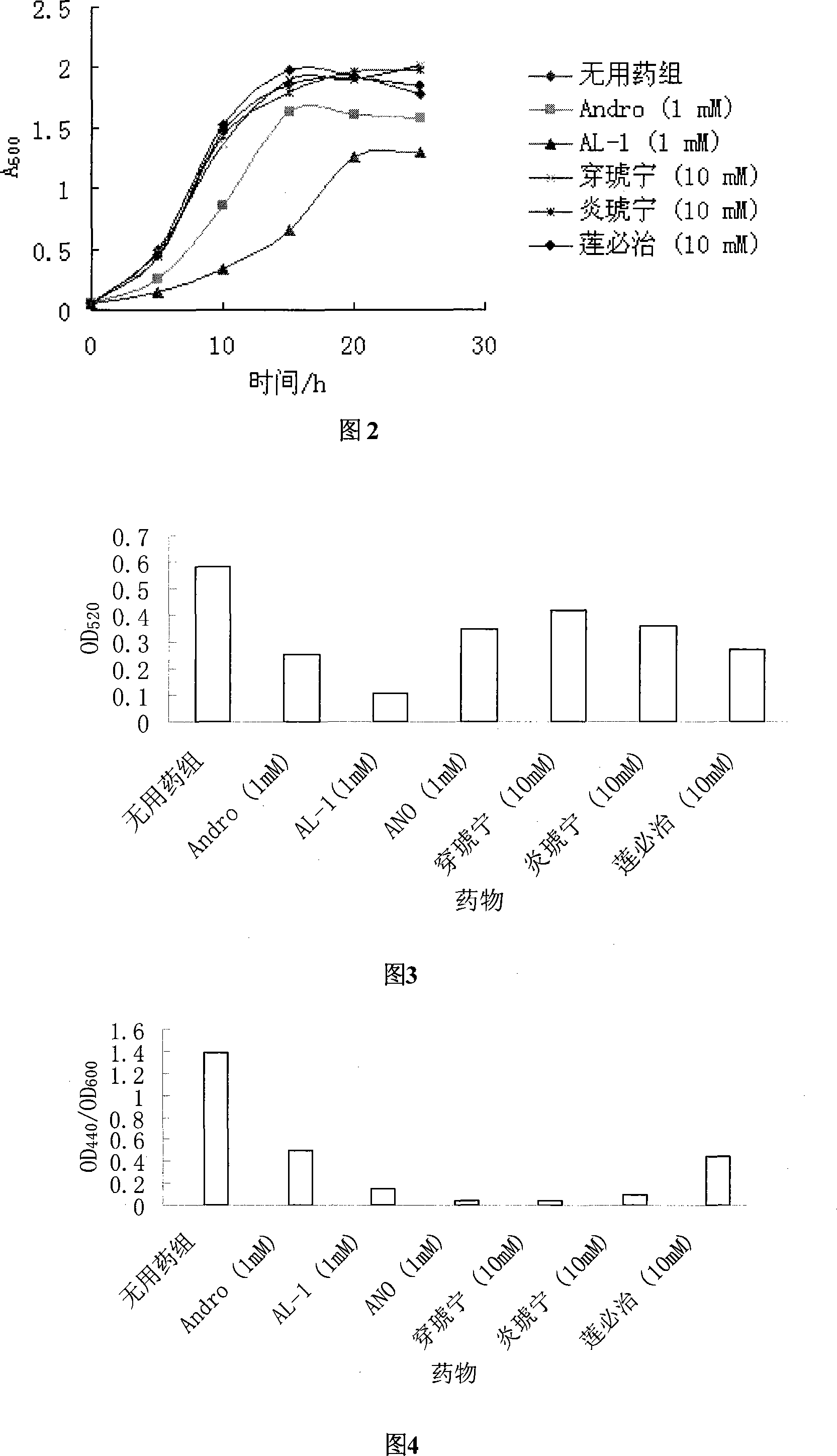
Image
Examples
Embodiment 1
[0122] Example 1. Preparation of 3,19-isopropylidene andrographolide 1
[0123] Dissolve andrographolide (150 mg, 0.43 mmol) in a mixed solution containing 2,2-dimethoxypropane (0.2 mL), pyridinium p-toluenesulfonate (3 mg) and toluene / DMSO (3 mL / 0.4 mL) , stirred at 80° C. for 1 hour, after the reaction was complete, cooled to room temperature, and quenched the reaction mixture with triethylamine (0.1 mL). The reaction mixture was diluted with toluene (20 mL), washed with water (3×5 mL), and the organic layer was washed with anhydrous Na 2 SO 4 After drying and concentration, the resulting white solid was washed with ether and filtered to obtain compound 1 (130 mg, 78%) (Srinivas et al., Bioorg. Med. Chem. Lett. 2004, 14, 4711-4717).
Embodiment 2
[0124] Example 2. Preparation of 14-lipoyl-3,19-isopropylidene andrographolide 2
[0125] Add ethyl chloroformate (0.11 mL) to α-lipoic acid (214 mg) CH 2 Cl 2 (8 mL) solution, add 0.21 mL of triethylamine, and stir at 0° C. for 1 h under the protection of nitrogen. Compound 1 (100 mg) was dissolved in CH 2 Cl 2 (8 mL), was added to the mixture, and stirred at room temperature for 2 days. Add CH after reaction 2 Cl 2 (30mL) diluted with NaHCO 3 Aqueous solution and water wash. Anhydrous NaSO for organic layer 4 Dry and concentrate in vacuo. The mixture was separated on a silica gel column to obtain compound 2 (90 mg, 61%) as a yellow crystalline powder. 1 H NMR (CDCl 3 , 400MHz): δ7.03(t, 1H, J=5.20Hz), 5.90(d, 1H, J=6.50Hz), 4.90(s, 1H), 4.20(dd, 1H, J=7.20, 17.20Hz) , 3.90 (d, 1H, J=11.99Hz), 1.42 (s, 3H), 1.37 (s, 3H), 1.21 (s, 3H), 0.81 (s, 3H). MS(ESI)[M+H] + m / z 578.
Embodiment 3
[0126] Example 3. Preparation of 14-lipoyl-3,19-dihydroxy-andrographolide AL-1
[0127] Compound 2 (100mg) was added into acidic aqueous solution (AcOH / H 2 O=7 / 3, 3 mL), stirred at room temperature for 1 h. The reaction mixture was added with water and NaHCO 3 In, use CH 2 Cl 2 (3 x 20 mL) extraction. Anhydrous NaSO for organic layer 4 Dry and concentrate in vacuo. The mixture was separated on a silica gel column to obtain compound AL-1 (42 mg, 45%) as a yellow crystalline powder. 1 H NMR (CDCl 3 , 400MHz): 7.03(t, 1H, J=5.20Hz), 5.93(d, 1H, J=6.50Hz), 4.88(s, H), 4.50(s, 1H), 4.24-4.17(dd, 1H, J=6.80, 18.40Hz), 3.34-3.32(d, 1H, J=10.79Hz), 1.34(s, 6H). HRMS[M] + m / z 538.2417, (calcd 538.2408).
[0128] Its synthetic reaction formula is as follows:
[0129]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com