High throughput proteomics
A protein and nucleotide sequence technology, applied in the field of high-throughput proteomics, can solve the problem of not being able to provide high-throughput methods for characteristic antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Prepare vector and insert
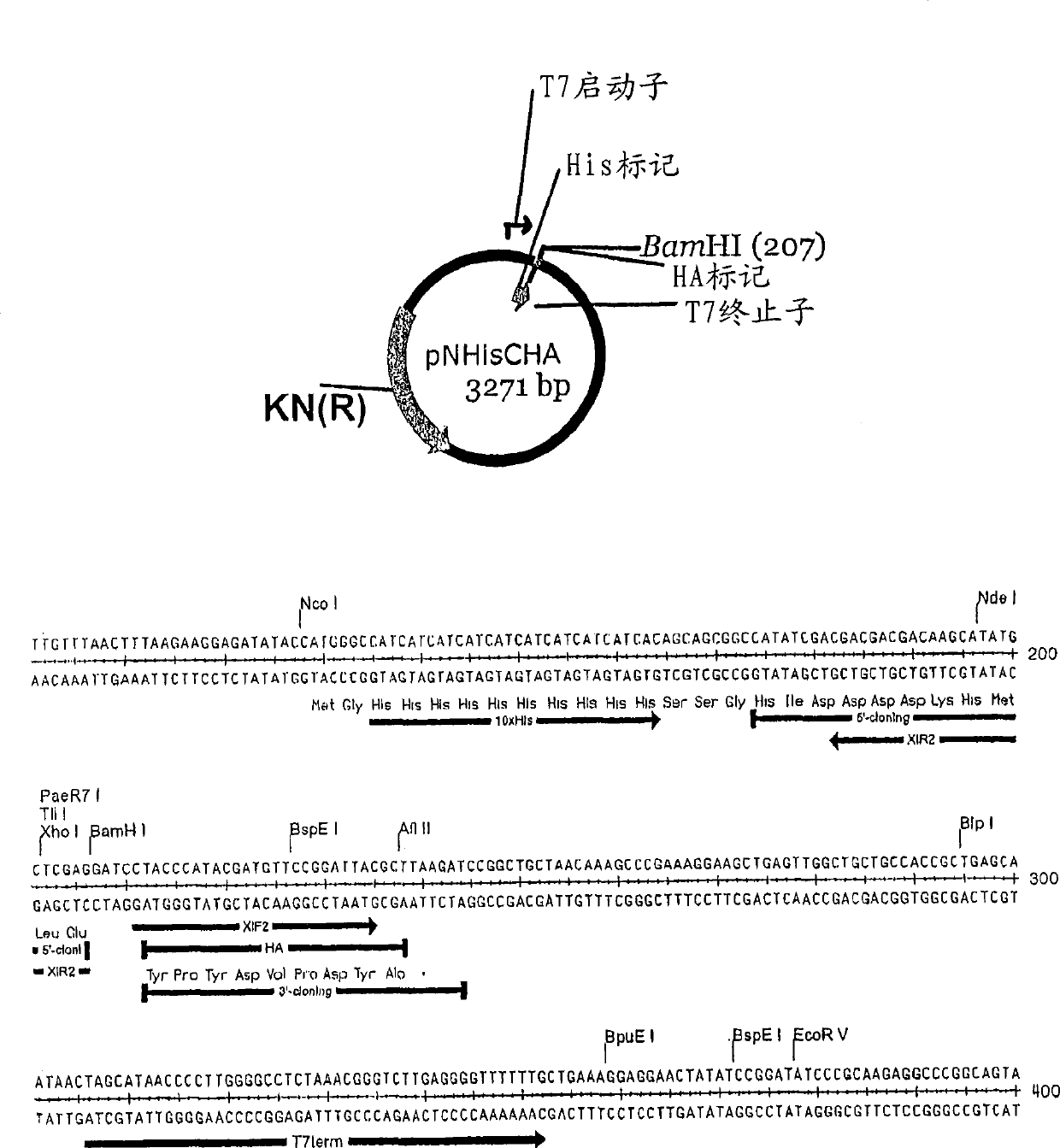
[0110] A linear T7 vector encoding an N-terminal histidine tag and a C-terminal HA tag was prepared by extensive restriction digestion followed by PCR; this procedure allows residual loops to be transformed when transformed without a complementary insert into chemically competent E. coli The amount of vector-like vectors and background colonies was reduced to close.
[0111] The plasmid used to make the linear recombinant vector pXT7 is shown in figure 1 . This vector contains a T7 promoter followed by an ATG start codon, a 10×histidine sequence, a spacer sequence before the first codon of the open reading frame to be cloned, a BamH1 site and a T7 terminator. The vector was double digested at the BamH1 site to eliminate residual circular vector, as incompletely digested vectors produced background colonies lacking the insert. This linearized vector is amplified by PCR to generate a stock of linear recombinant vectors. Each batch of lin...
Embodiment 1A
[0117] Example 1A : Applying these methods to vaccinia virus required preparation of primers for 213 genes, of which 211 PCR products were isolated (>99%). All 211 were cloned and 181 of the products were sequenced; 93% (169 of 181) provided predicted sequences.
Embodiment 1B
[0118] Example 1B : Similarly, applying this method to Plasmodium falciparum requires the preparation of primers for 720 genes. 462 PCR products (64%) were thus obtained, resulting in 266 clones (58%). A group (63) of these were sequenced and 97% yielded the expected sequence.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com