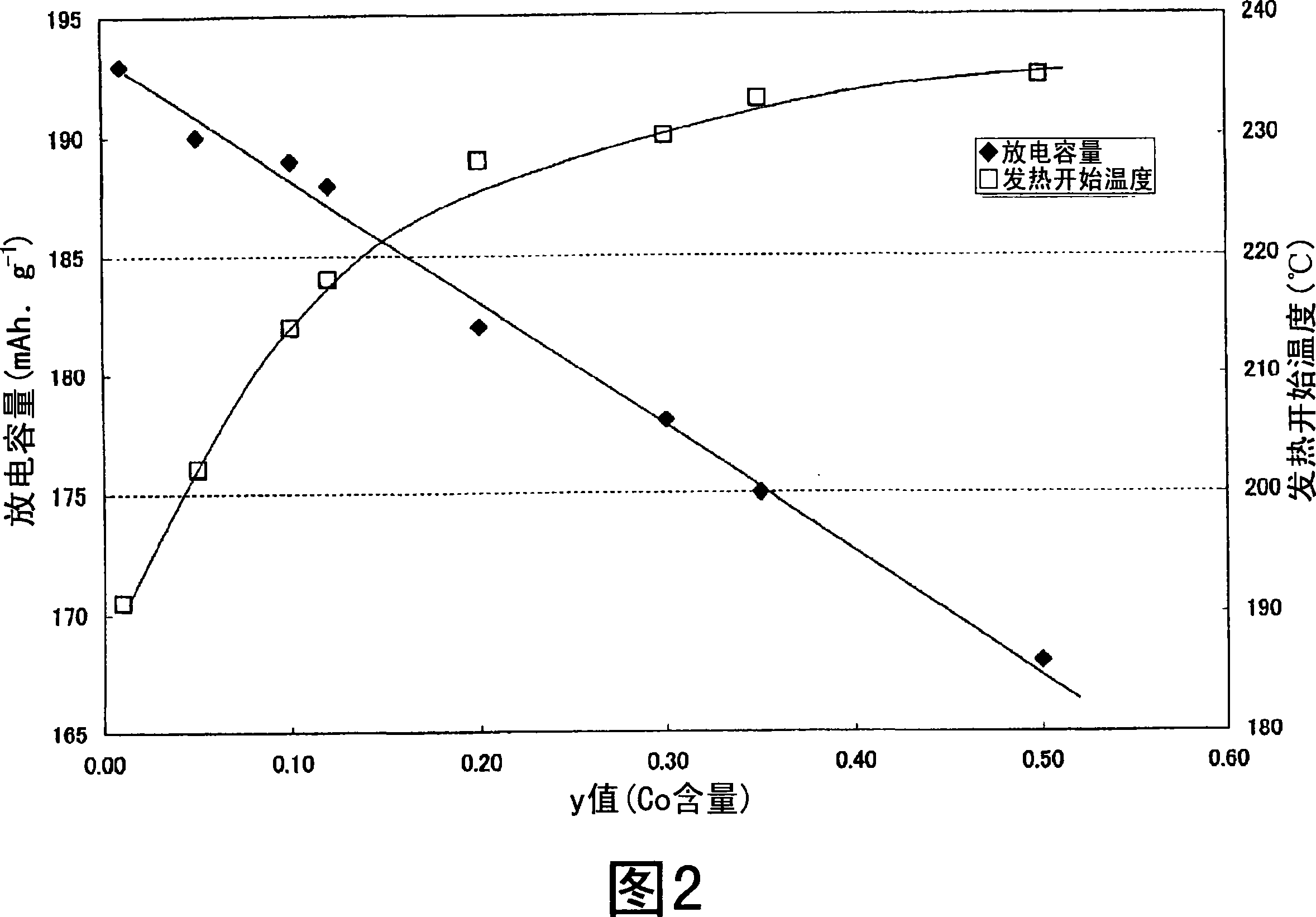
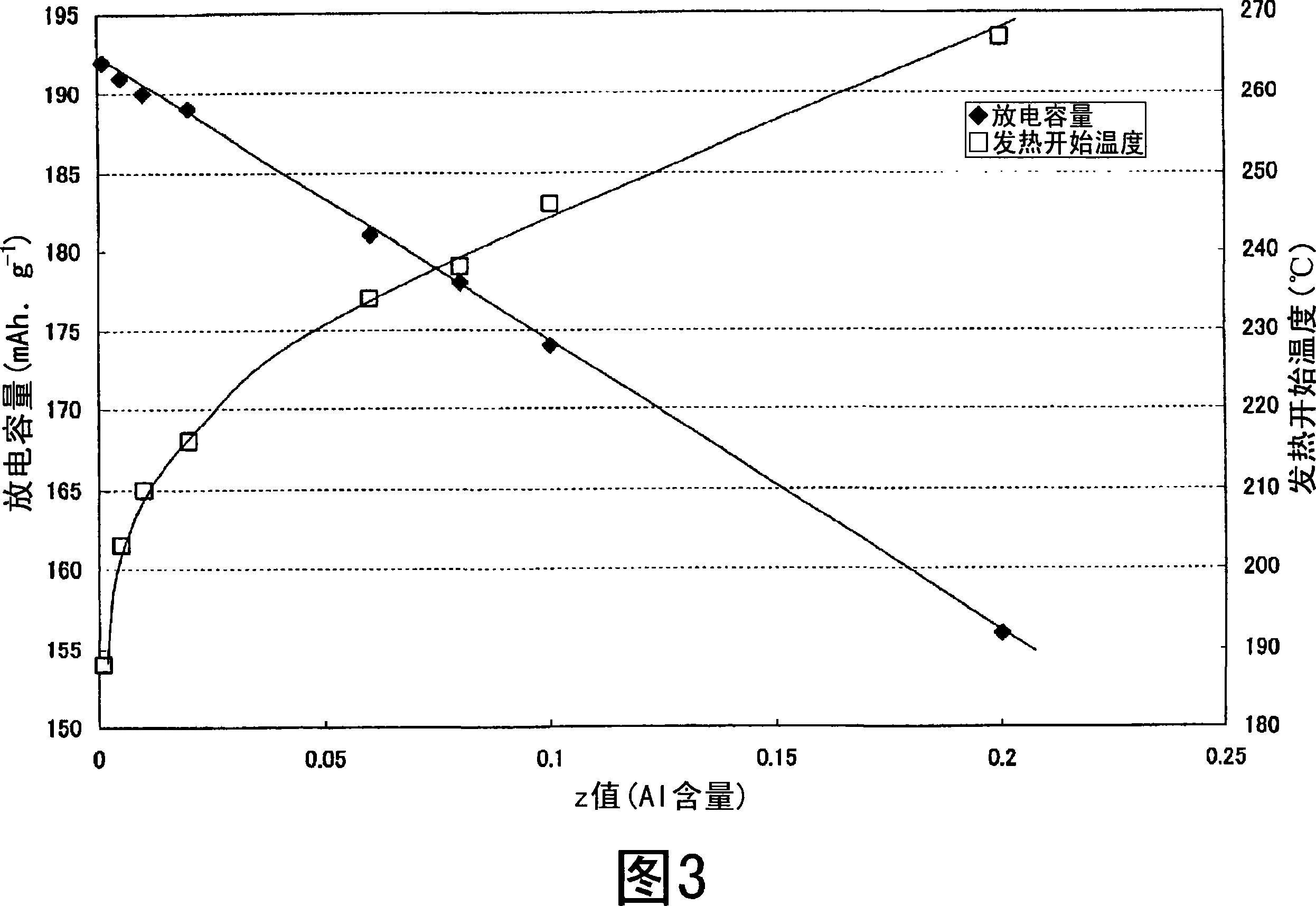
Nonaqueous electrolyte secondary battery
A non-aqueous electrolyte, secondary battery technology, applied in non-aqueous electrolyte storage batteries, secondary batteries, battery electrodes, etc., can solve the problems of not seeing sufficient improvement, not finding cycle characteristics and high temperature storage characteristics, etc. Suppression of side reactions, balance between cycle characteristics and high-temperature storage characteristics, and high-capacity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Composite oxides having compositions and physical properties of Nos. 1 to 31 shown in Tables 1 to 4 were prepared as positive electrode active materials by the following method, and Batteries 1 to 31 were fabricated using the positive electrode active material.
[0095] (i) Preparation of positive electrode active material
[0096] Process a
[0097] Prepare and dissolve nickel sulfate, cobalt sulfate, element M 1 salt and element M 2 salt solution of metal salts. The concentration of nickel sulfate in the above-mentioned metal salt aqueous solution is set as 1mol / L, and the concentration of other salts is adjusted appropriately according to Table 1.
[0098] With stirring, the above metal salt aqueous solution was maintained at 50° C., and an aqueous solution containing 30% by weight of sodium hydroxide was added dropwise thereto to bring the pH to 12, whereby hydroxide was precipitated. The hydroxide precipitate was filtered, washed with water, and dried in air. ...
Embodiment 2
[0154] Except that the composition and physical properties of the positive electrode active material were changed to No. 38-45 shown in Table 7 and Table 8, the composite oxide was prepared in the same manner as in Example 1, and a battery was fabricated in the same manner as in Example 1 using the composite oxide. 38~45.
[0155] Table 7
[0156] Li x Ni 1-y-z-v-w co y Al z m 1 v m 2 w o 2
No.
M 1
M 2
x
y
z
v
w
v / w
comparative example
38
mn
Mg, Ca
1
0.15
0.03
0.00005
0.005
0.01
Example
39
mn
Mg, Ca
1
0.15
0.03
0.0001
0.005
0.02
Example
40
mn
Mg, Ca
1
0.15
0.03
0.0005
0.005
0.1
Example
41
mn
Mg, Ca
1
0.15
0.03
0.0015
0.005
0.3
Example
42
mn
Mg, Ca ...
Embodiment 3
[0166] Except that the composition and physical properties of the positive electrode active material were changed to No. 46-53 shown in Table 9 and Table 10, the composite oxide was prepared in the same manner as in Example 1, and a battery was fabricated in the same manner as in Example 1 using the composite oxide. 46-53.
[0167] Table 9
[0168] Li x Ni 1-y-z-v-w co y Al z m 1 v m 2 w o 2
No.
M 1
M 2
x
y
z
v
w
v / w
comparative example
46
mn
Mg, Ca
1
0.15
0.03
0.005
0.00005
100.00
Example
47
mn
Mg, Ca
1
0.15
0.03
0.005
0.0001
50.00
Example
48
mn
Mg, Ca
1
0.15
0.03
0.005
0.0005
10.00
Example
49
mn
Mg, Ca
1
0.15
0.03
0.005
0.0015
3.33
Example
50
mn
M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com