Reaction system and synthetic method for producing chloropropyl triethoxy silicane continuously
A technology of chloropropyltriethoxysilane and chloropropyltrichlorosilane, which is applied in the field of reaction devices for synthesizing chloropropyltriethoxysilane, can solve the problem of containing more hydrogen chloride, long synthesis reaction cycle, and production cost Advanced problems, to achieve the effect of low production cost, short reaction cycle and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
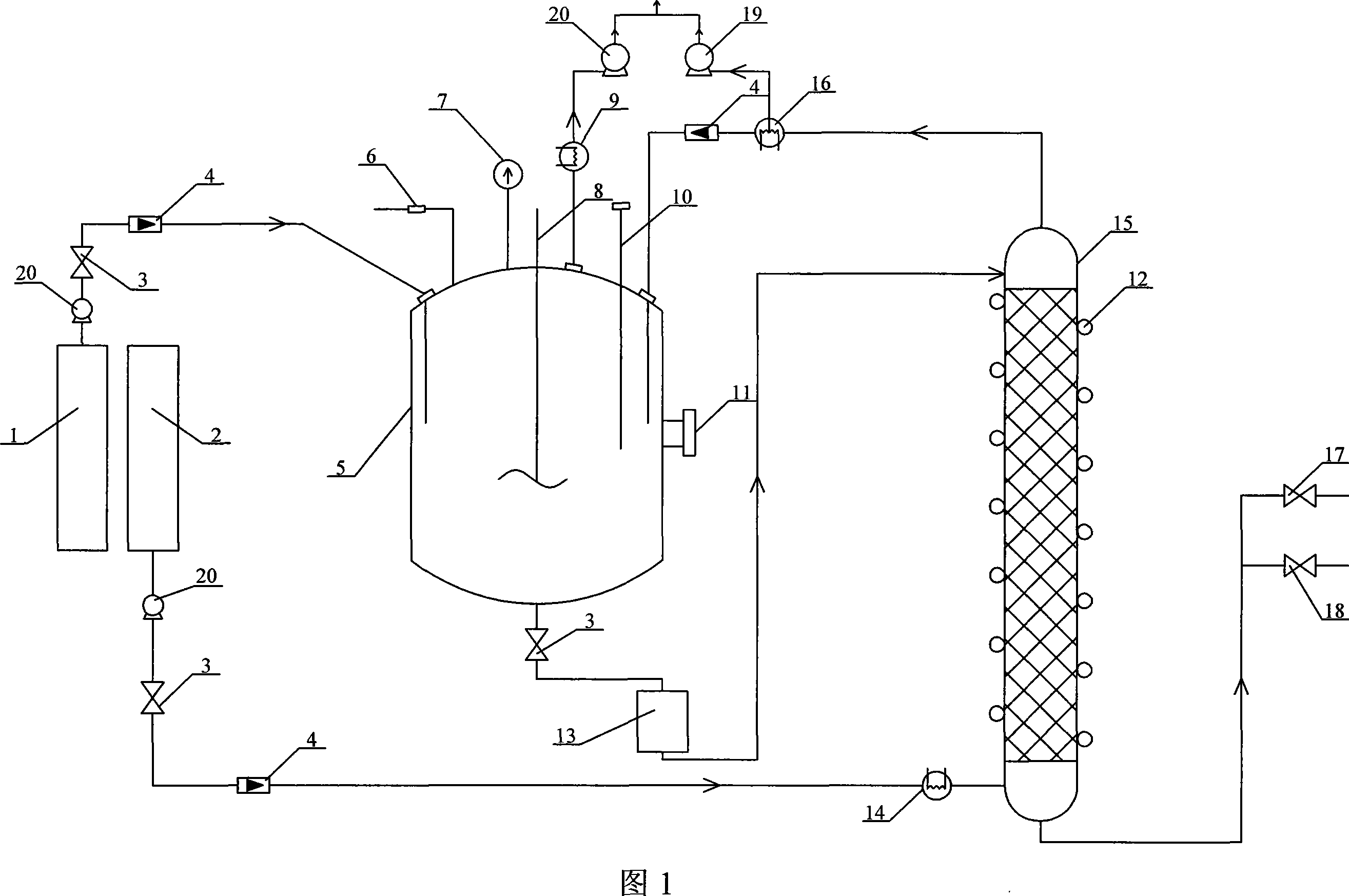
[0010] Specific embodiment one: (referring to Fig. 1) the reaction system of this embodiment consists of chloropropyl trichlorosilane storage tank 1, ethanol storage tank 2, esterification reactor 5, safety valve 6, pressure gauge 7, agitator 8, First heat exchanger 9, thermocouple 10, heater and temperature controller 11, temperature control resistance wire 12, primary product storage tank 13, second heat exchanger 14, stripping tower 15, third heat exchanger 16, Outlet valve 17, sampling valve 18, the first ceramic pump 19 and the second ceramic pump 20 for air extraction; port is communicated, the liquid outlet at the bottom of the ethanol storage tank 2 is communicated with the liquid inlet of the second heat exchanger 14, and the gas outlet of the second heat exchanger 14 is communicated with the air inlet of the stripping tower 15 bottom, and the gas stripping tower 15 The air outlet at the top communicates with the air inlet of the third heat exchanger 16, the air outle...
specific Embodiment approach 2
[0011] Embodiment 2: The packing in this embodiment is ceramic Pall ring packing, Raschig ring, ceramic saddle ring packing or saddle packing. Other structures and connection methods are the same as those in the first embodiment.
specific Embodiment approach 3
[0012] Specific embodiment three: this embodiment adopts the reaction system in specific embodiment one to synthesize chloropropyl triethoxysilane, and the synthetic method is as follows: the purity is 99% chloropropyl trichlorosilane and dehydrated alcohol by 1: 3.15 Feed at a molar ratio of ~3.3, the reaction pressure is maintained between -0.03 ~ -0.09MPa, the reaction cycle is 3 ~ 3.5h, add absolute ethanol continuously during the reaction cycle, and the reaction temperature increases from 25°C with the addition of absolute ethanol The temperature is raised to 50°C; during the reaction, chloropropyltrichlorosilane is directly added to the esterification reactor 5, and the absolute ethanol is heated to 90-115°C through the second heat exchanger 14 and then enters the stripping tower 15, and the initial product After the mass transfer and heat transfer, the top of the stripper 15 enters the esterification reactor 5 through the third heat exchanger 16 to undergo an esterificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com