Lactone compound of sweetsop connected through amido bond, synthetic method and application
A technology of annua lactone and a compound, which is applied in the field of synthesis of chiral annua lactone analogs, can solve the problems of poor water solubility, low synthesis efficiency, limited research progress of patent medicines and the like, and achieves a simple preparation method and high anti-cancer properties. active effect
Inactive Publication Date: 2010-12-22
SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In this series of compounds, the carbon skeleton of the molecule is constructed through the connection of ether bonds, which makes its synthesis efficiency not high; and the water solubility of this series of compounds is poor, which will limit the progress of its later drug research
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
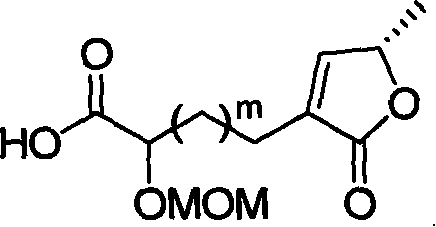
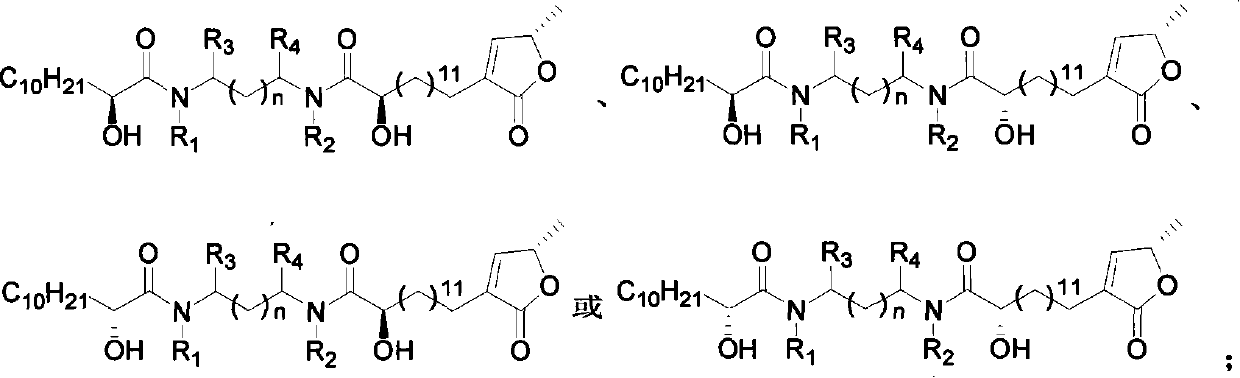
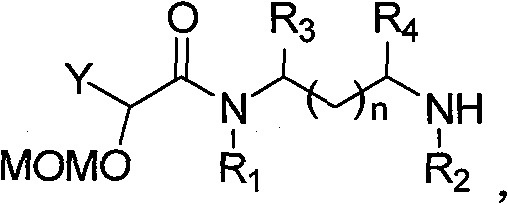
This invention relates to an acetogenin compound linked by amide bonds, its synthesis method and its application. The acetogenin compound is an optically active or racemized compound, whose structural formula is shown in this invention, wherein, Y is C6-20 alkyl; n is 0-3; m is 7-19; R1, R2, R3 and R4 are the same or not, and are H or C1-10 alkyl; when n is 0-2, R3 and R4 are H, Cl, C1-10 alkyl or combined to form fiver-, six- or seven-membered ring shape; when n is 0, R1 and R2 are C1-10 alkyl or combined to form fiver-, six- or seven-membered ring shape. The acetogenin compound has high anticancer activity, and IC50 (mu.M) is lower than 0.04 to breast cancer (MDA-MB-435S). The can be manufactured into anticancer drugs. The synthesis method is simple, and suitable for industrial production.
Description
technical field The invention relates to a lactone compound, in particular to a synthesis method and application of a chiral anemone lactone analog connected by an amide bond. Background technique Currently, cancer has become the greatest threat to human health, and its incidence is increasing year by year. Cancer is responsible for more than 20% of all deaths each year, a figure that exceeds the combined number of deaths from AIDS, tuberculosis and malaria. Traditional cancer treatment methods, such as surgical resection, radiotherapy and chemotherapy, have certain defects, either incomplete treatment or severe side effects. With the development of modern organic separation identification and synthesis technology, many chemists are committed to the separation and identification of natural compounds with high anti-tumor activity and selectivity from natural products, and obtain this natural compound with relatively high content through organic synthesis. Low compounds, and...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D307/58A61K31/365A61K31/496A61P35/00
CPCY02P20/55
Inventor 姚祝军刘海侠张焕明
Owner SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com