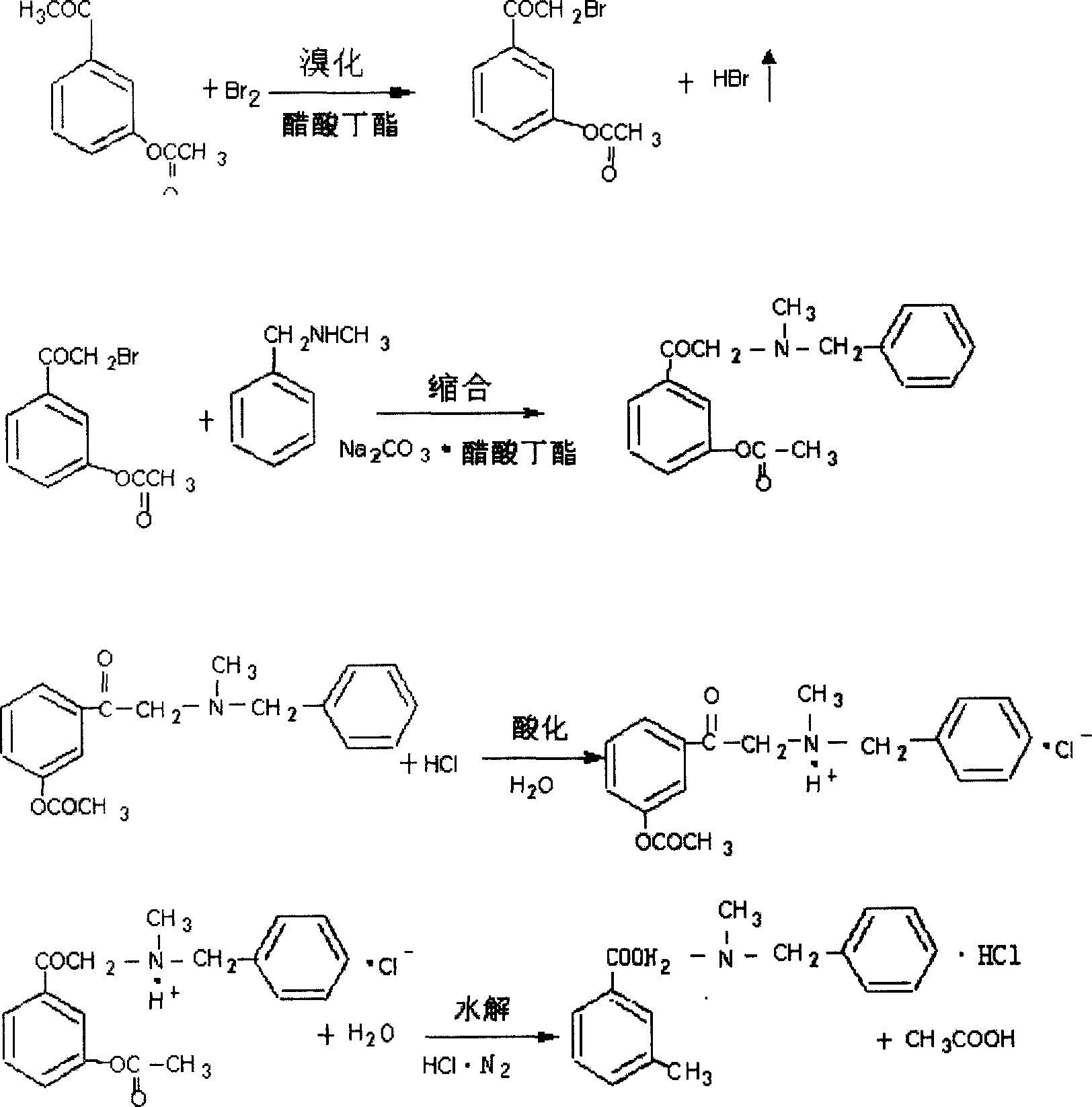
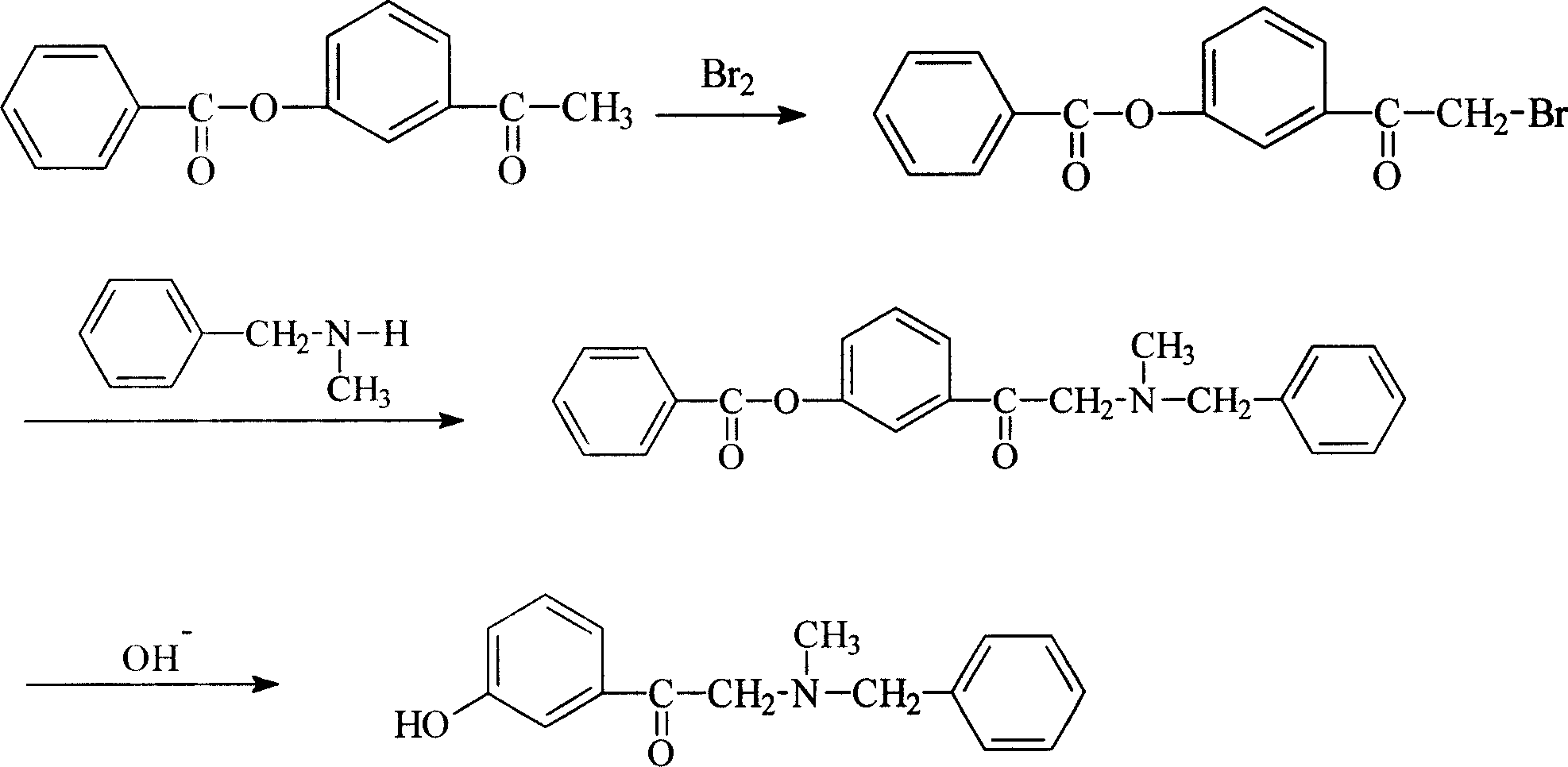
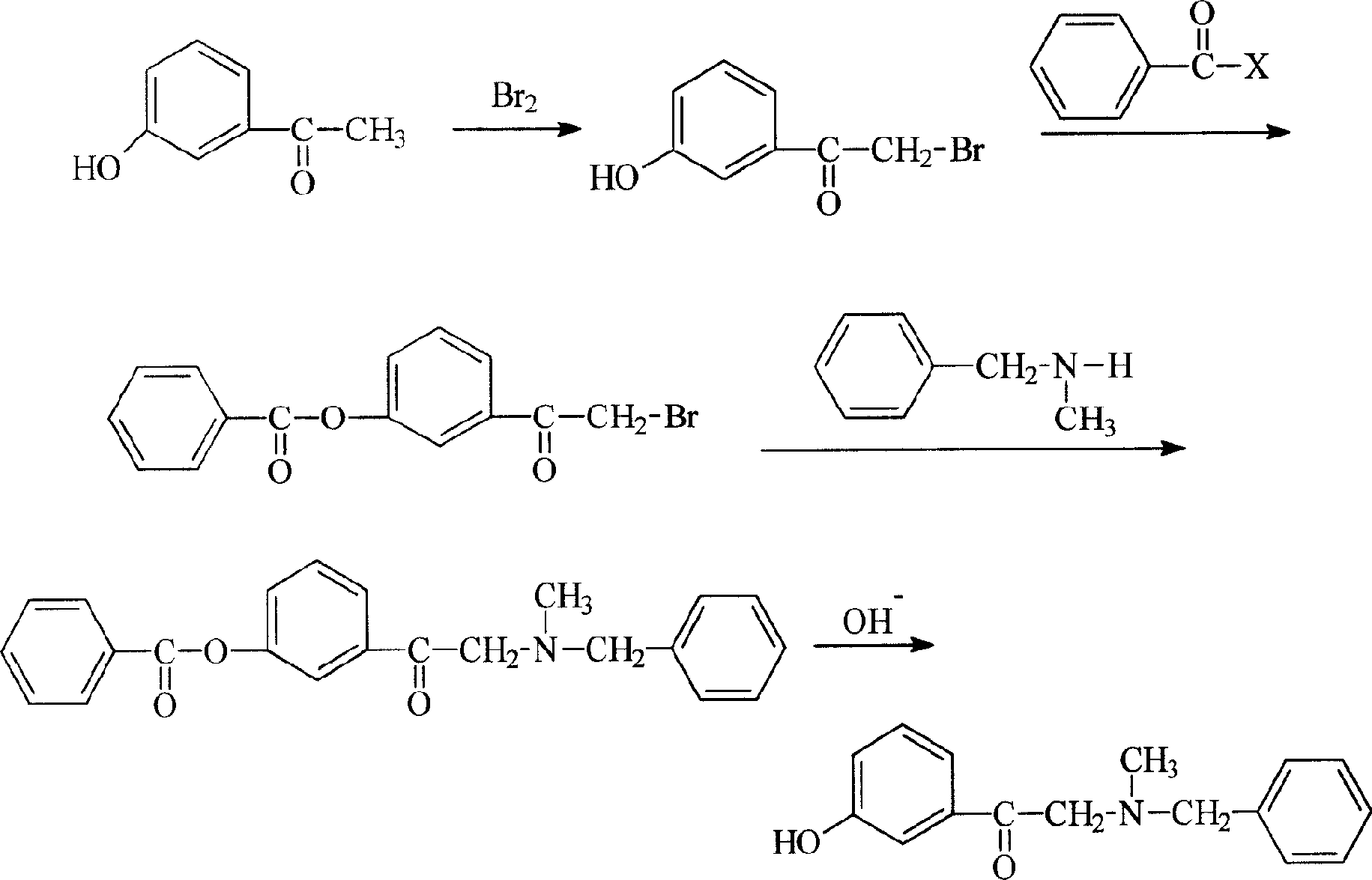
Process of synthesizing alpha-(N-methyl-N-benzylamin)-3-hydroxy acetophenone hydrochloride
A technology of hydroxyacetophenone hydrochloride and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as low product purity, achieve simplified process, simple and easy environmental management, The effect of improving the production environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040] Feeding amount of wet crude product (single pot)
[0041] Butyl acetate: 300kg (one barrel) Nitrogen: 3 bottles
[0042] 3-Acetoxyacetophenone: 178kg
[0043] Bromine: 160kg
[0045] N-Methylbenzylamine: 121kg Butyl acetate: 300kg
[0046] Hydrochloric acid: 100kg
[0048] 1. Put 300kg of butyl acetate and 178kg of 3-acetoxyacetophenone into a 500L reaction pot, stir for 15 minutes, and add dropwise 160kg of bromine previously pumped into the high level tank at an internal temperature of 20°C. Use a low-vacuum four-stage low-cooled water absorption device to recover hydrogen bromide gas, dropwise, keep warm for 5 minutes, cool to 20°C, and discharge to a 1000L reaction pot (wherein there is 100kg of water at 0°C), and continue stirring after discharging 5 minutes, let stand for 30 minutes, separate the lower water layer, extract the water layer with 100kg hydrochloric acid recovered butyl acetate once...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com