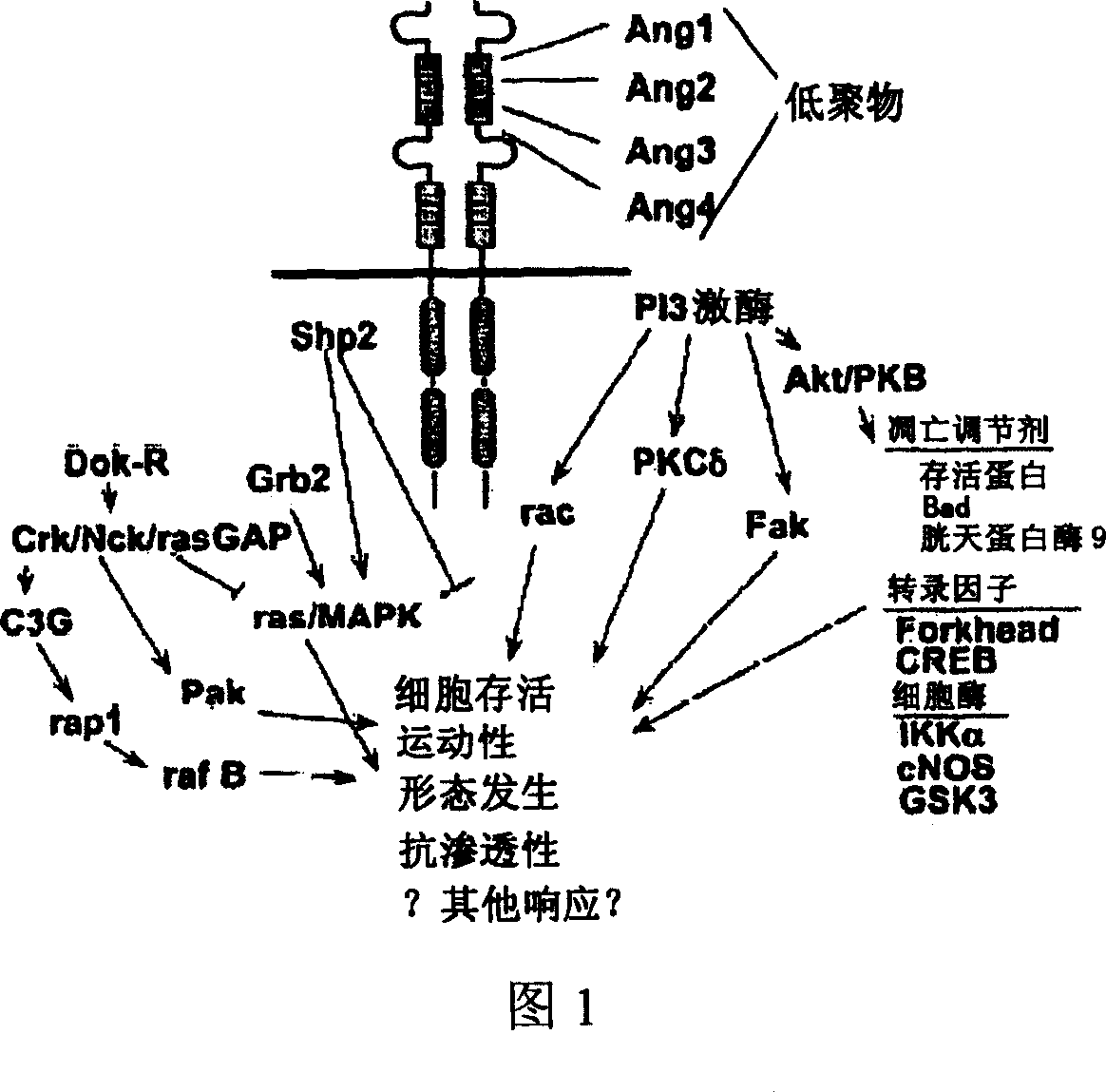
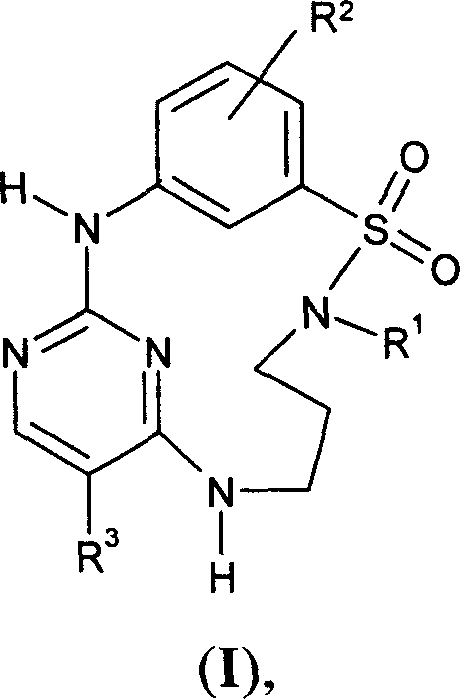
Sulfonamido-macrocycles as tie2 inhibitors
A technology of alkyl and hydroxyl, which is applied in the field of preparing sulfonylamino macrocycles, and can solve the problems of selective small dose tolerance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example A
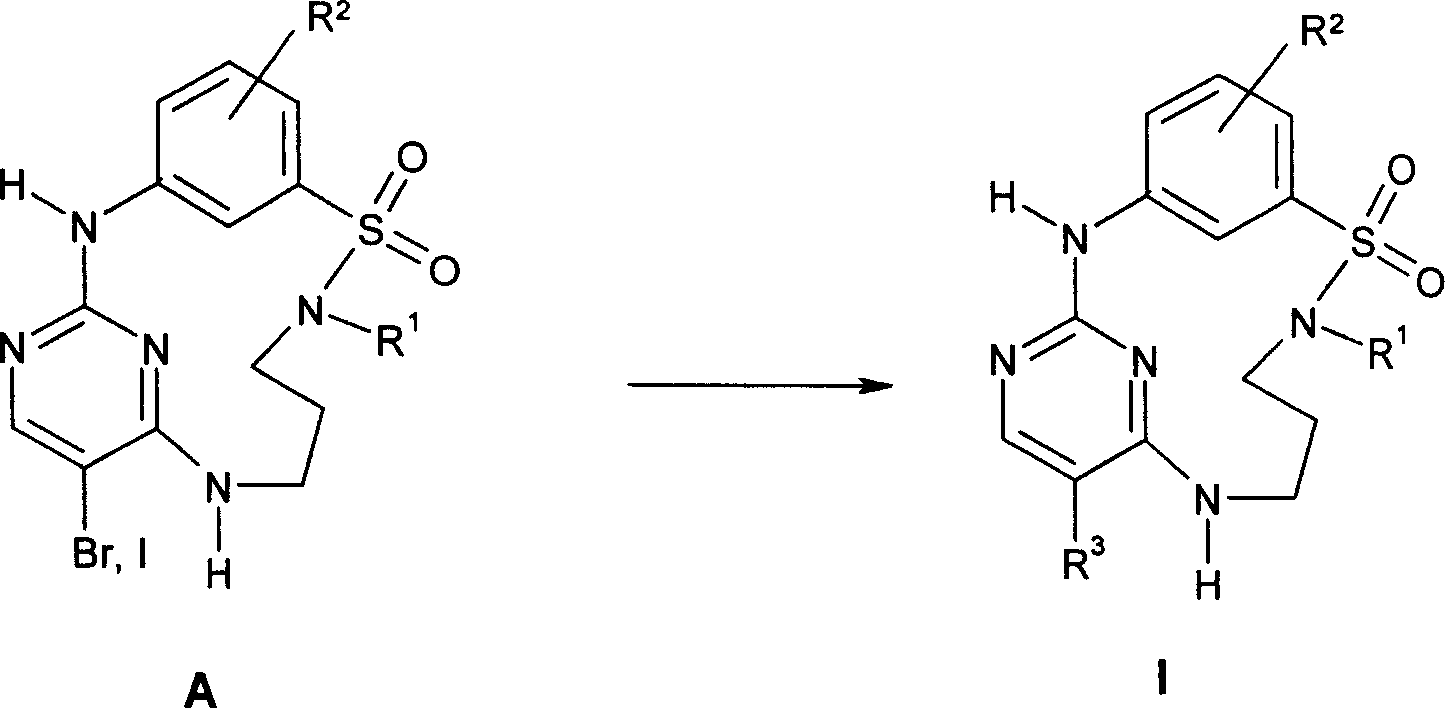
[0100] The synthesis of halogenated macrocycle A is described in WO 2004 / 026881 A, exemplified herein as Preparation A for brominated macrocycle A, and Preparation B for iodinated macrocycle A.
[0101] Preparation A: 1 5 -Bromo-4-thia-2,5,9-triaza-1(2,4)-pyrimidina-3(1,3)-phenheteronone-phane-4,4-dioxide preparation
[0102]
[0103] Method A
[0104] 200 mg (0.48 mmol) of 3-amino-N-[3-(5-bromo-2-chloro-pyrimidin-4-ylamino)-propyl]-benzenesulfonamide in acetonitrile / A water / 2-butanol (9.0ml / 1.0ml / 0.3ml) solution was added to a refluxing mixture of acetonitrile / water / 4 molar hydrochloric acid in dioxane (45ml / 5ml / 0.6ml). After another 3 hours at reflux, the oil bath was turned off and the reaction solution was stirred overnight at room temperature. The precipitate formed was filtered off, washed with water and dried in vacuo. 112 mg (0.31 mmol) of product are obtained. The filtrate was concentrated by evaporation in a rotary evaporator. The formed precipitate was w...
preparation example B
[0114] Preparation Example B: 1 5 -Iodo-4-thia-2,5,9-triaza-1(2,4)-pyrimidina-3(1,3)-phenheteronone-phane-4,4-dioxide preparation
[0115]
[0116] Over 3 hours, 2.34 g (5.00 mmol) of 3-amino-N-[3-(5-iodo-2-chloro-pyrimidin-4-ylamino)-propyl]-benzenesulfonamide in acetonitrile was injected via a syringe pump A solution of water / 2-butanol (94 mL / 10.4 mL / 3.1 mL) was added to a refluxing mixture of acetonitrile / water / 4 molar hydrochloric acid in dioxane (470 mL / 52 mL / 6.2 mL). After another 3 hours at reflux, the heating of each oil bath was turned off and the reaction solution was stirred overnight at room temperature. The formed precipitate was filtered off, washed with acetonitrile and dried in vacuo to afford 1.71 g (79% yield) of the desired product.
[0117] 1 H-NMR (DMSO, 300MHz): 10.81 (s, 1H), 9.02 (s, 1H), 8.30-8.38 (m, 1H), 8.27 (s, 1H), 7.82 (t, 1H), 7.43-7.56 ( m, 2H), 7.29-7.40 (m, 1H), 3.38-3.52 (m, 2H), 3.21-3.36 (m, 2H), 1.72-1.90 (m, 2H).
[0118] ESI-...
preparation example 1 to 43
[0126] General Procedure 1 (GP1): Suzuki Coupling
[0127] (Typical range: 0.25mmol)
[0128] At room temperature, with the corresponding organoboron compound (1.25eq.), K 2 CO 3 (2.5 eq., either as a solid or as a 2M aqueous solution), and POPd (2.5-5 mol-%) were treated with a DMF (8 mL / mmol halide) solution of the corresponding macrocyclic halide. The stirred resulting mixture was placed in an oil bath preheated to 100°C. The progress of the reaction was monitored by TLC, in case the macrocyclic halide was not completely converted after 2 h, additional portions of POPd and organoboron compound were added, followed by further stirring at 100 °C. After cooling to room temperature, water was added and the resulting suspension was stirred for 30 min. The crude product is isolated by vacuum filtration, dried in vacuo, purified by column chromatography, then optionally triturated with methanol and / or subjected to preparative HPLC (e.g. YMC Pro C 18 RS 5μ, 150×20mm, 0.2% NH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com