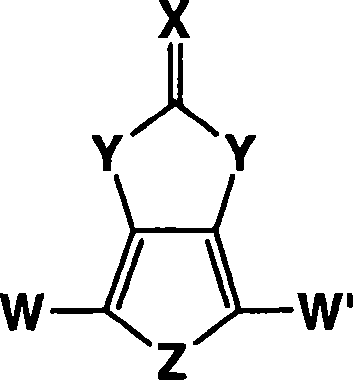
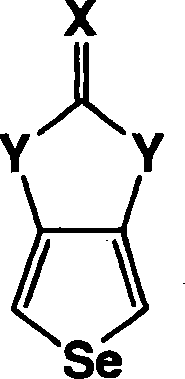
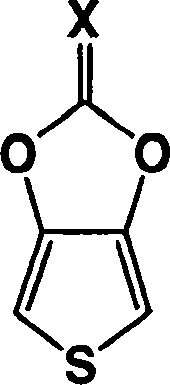
Heterocyclic fused imidazolone, dioxolone, imidazolethione and dioxolethione monomers
A technology of monomers and compounds, applied in the field of monomers for generating conductive polymers, can solve the problems of dangerous storage and disposal, difficult operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The method of the present invention can include the synthesis of 1H-thieno[3,4-d]imidazolin-2(3H)-one in a single reaction mixture. The synthesis method of 1H-thieno[3,4-d]imidazolin-2(3H)-one according to the present invention includes the mechanism shown below:
[0057] This first reactant compound, second reactant compound, solvent and base are present in the reaction mixture. In the above reaction, 3,4-diaminothiophene dihydrochloride is shown as sodium carbonate (Na 2 CO 3 ) Reacts in the presence of a base to form 3,4-diaminothiophene, which reacts with the second reactant compound of urea shown above to form 1H-thieno[3,4-d]imidazoline-2(3H)- ketone.
[0058] The method of the present invention also includes the synthesis of imidazolinone selenophene derivatives. In this embodiment of the present invention, the reaction of 3,4-diaminoselenophene dihydrochloride is as follows:
[0059] In the above reaction, 3,4-diaminoselenophene dihydrochloride is shown as sod...
Embodiment 1
[0078] According to the method of the present invention, the compound 1H-thieno[3,4-d]imidazolin-2(3H)-one is prepared in a single reaction mixture. In Example 1, triethylamine was used as a base to release 3,4-diaminothiophene from dihydrochloride. Urea is used as the second reactant compound containing a carbonyl group.
[0079] Set the quantity to 0.1922g (1.028×10 -3 mol) 3,4-Diaminothiophene dihydrochloride ("DAT-HCl") and 0.1866g (3.11×10 -3 mol; 3.02 equivalents based on DAT-HCl) Urea was added to a 50 mL three-necked flask equipped with magnetic stirring and reflux condenser. The flask was purged with nitrogen for 15 minutes, after which 20 mL of 1,3-dimethyl-2-imidazolidinone ("DMI") was added with a syringe under stirring. Heat the contents to 50℃, at this time add 0.45mL (0.327g, 3.23×10 -3 mol; 3.14 equivalents based on DAT-HCl) triethylamine. The temperature of the flask was raised to 115-120°C and kept in this temperature range for 4 hours. After cooling and filte...
Embodiment 2
[0082] According to the method of the present invention, 1H-thieno[3,4-d]imidazolin-2(3H)-one is prepared in a single reaction mixture. Example 2 uses 4-(dimethylamino)pyridine, an alternative base of Example 1, as the base to release 3,4-diaminothiophene from the dihydrochloride. In addition, Example 2 uses 1,1'-carbonyldiimidazole as the second reactant compound.
[0083] Set the quantity to 0.1934g (1.034×10 -3 mol) 3,4-Diaminothiophene dihydrochloride (DAT-HCl), 0.4862g (3.001×10 -3 mol; 2.90 equivalents based on DAT-HCl) 1,1'-carbonyldiimidazole, and 0.3957g (3.243×10 -3 mol; 3.14 equivalents based on DAT-HCl) 4-(dimethylamino)pyridine was added to a 50 mL three-necked flask equipped with magnetic stirring and reflux condenser. The flask was purged with nitrogen for 15 minutes, and then 20 mL of 1,3-dimethyl-2-imidazolidinone (DMI) was added with a syringe while stirring. The temperature of the flask was raised to 115-120°C and kept in this temperature range for 4 hours. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com