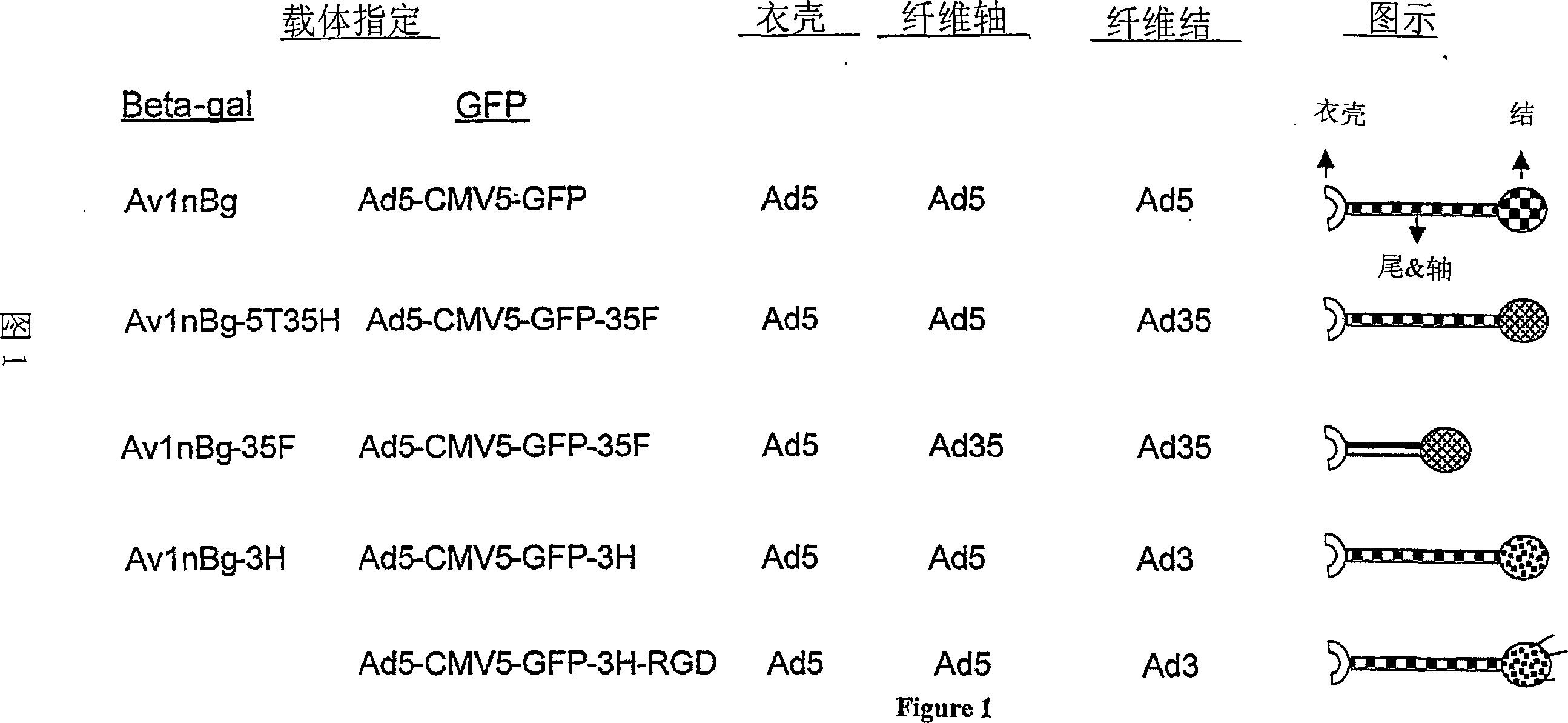
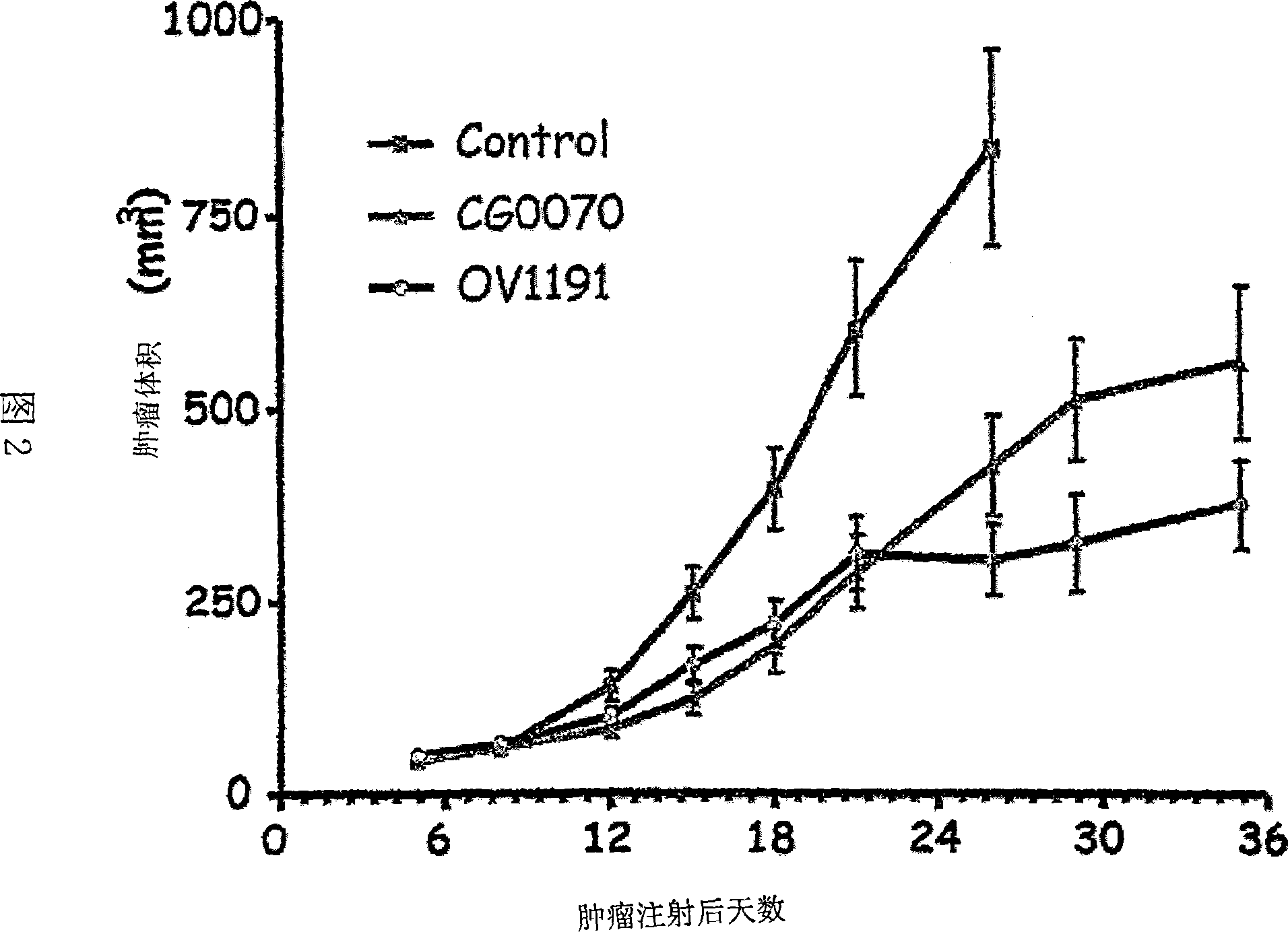
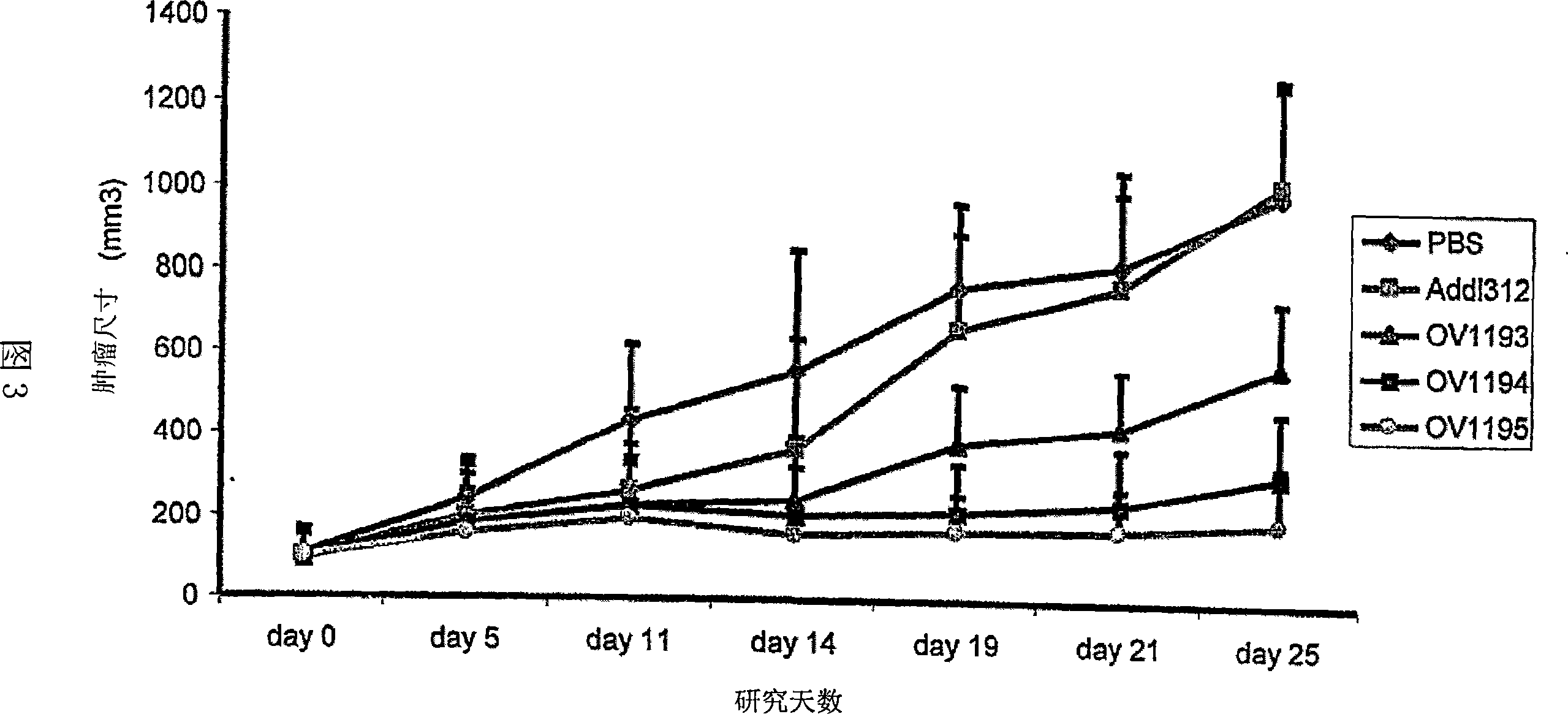
Fiber-modified adenoviral vectors for enhanced transduction of tumor cells
A tumor cell and adenovirus technology, applied in the field of adenovirus vectors, can solve the problem of low efficiency of primary tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0153] Preparation and production of adenoviral vectors
[0154] Standard systems for generating adenoviral vectors for insert expression are known in the art and are available from commercial sources, such as from Clontech (Palo Alto, CA) (Clontechniques (January 2000) pp. 10-12). Adeno-X TM Expression system, Adenovator from Qbiogene (Carlsbad, CA) TM Adenoviral vector system and AdEasy TM .
[0155]For convenience, plasmids providing the necessary parts of the adenovirus are available. Plasmid pXC.l (McKinnon (1982) Gene 19:33-42) contains the wild-type left-hand end of Ad5. PBHG10 (Bett et al. (1994); Microbix Biosystems Inc., Toronto) provides a right-hand end of Ad5 with a deletion at E3. PBHG11 provides a larger E3 deletion with an additional 0.3 kb deleted (Bett et al 1994). Alternatively, the use of pBHGE3 (Microbix Biosystems, Inc.) provides the right-hand end of Ad5 with the full length of E3.
[0156] To address early genes, the transcription start site of A...
Embodiment 1
[0250] Example 1: Human Tumor Cell Lines and Cell Culture
[0251] The human head and neck cancer lines and human melanoma cell series used in the examples described herein are listed in Table 1.
[0252] cell line
[0253] The human head and neck cancer lines and human melanoma cell lines listed in Table 1 were cultured in RPMI 1640 medium containing 10% FBS. The human prostate cancer cell line, PC3M-2AC6, was cultured in RPMI 1640 medium containing 10% FBS. The human prostate cancer cell line, LNCaP (ATCC, CRL-10995). The human prostate cancer cell line, PC-3 (ATCC, CRL-1435), was cultured in RPMI supplemented with 10% FBS and L-glutamine (2 mM).
Embodiment 2
[0254] Example 2: Construction of E1-deficient, GFP-expressing fiber chimeric Ad5 vector
[0255] To generate fiber chimera-based Ad5 vectors, the shuttle plasmid, pAd5LtRt-Smal, was first constructed as described below. First, the left-hand end of Ad5DNA (bp 1-1009bp) was amplified by PCR with two primers;
[0256] Primer 1 (5'-GAATTCTAGGGATAACAGGGTAATCATCATCAATAATACCTT-3'; SEQ ID NO: 17) and Primer 2 (5'-CCCGGGGTGCTCCACATAAATCT-3'; SEQ ID NO: 18). The right end of Ad5 DNA was amplified using a second set of primers designated primer 3 (5'-AAGCTTTAGGGATAACAGGGTAATCATCATCAATAATACCTT-3'; SEQ ID NO: 19) and primer 4 (5'-CCCGGGGGAATACATACCCGCAGG-3'; SEQ ID NO: 20) 580bp sequence. A recognition sequence for I-SceI was incorporated into primers 1 and 3. The first PCR product was digested with EcoRI and Smal and the second PCR product was digested with HindIII and Smal. The resulting fragment was gel purified and cloned by three-way ligation into the EcoRI and HindIII sites of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com