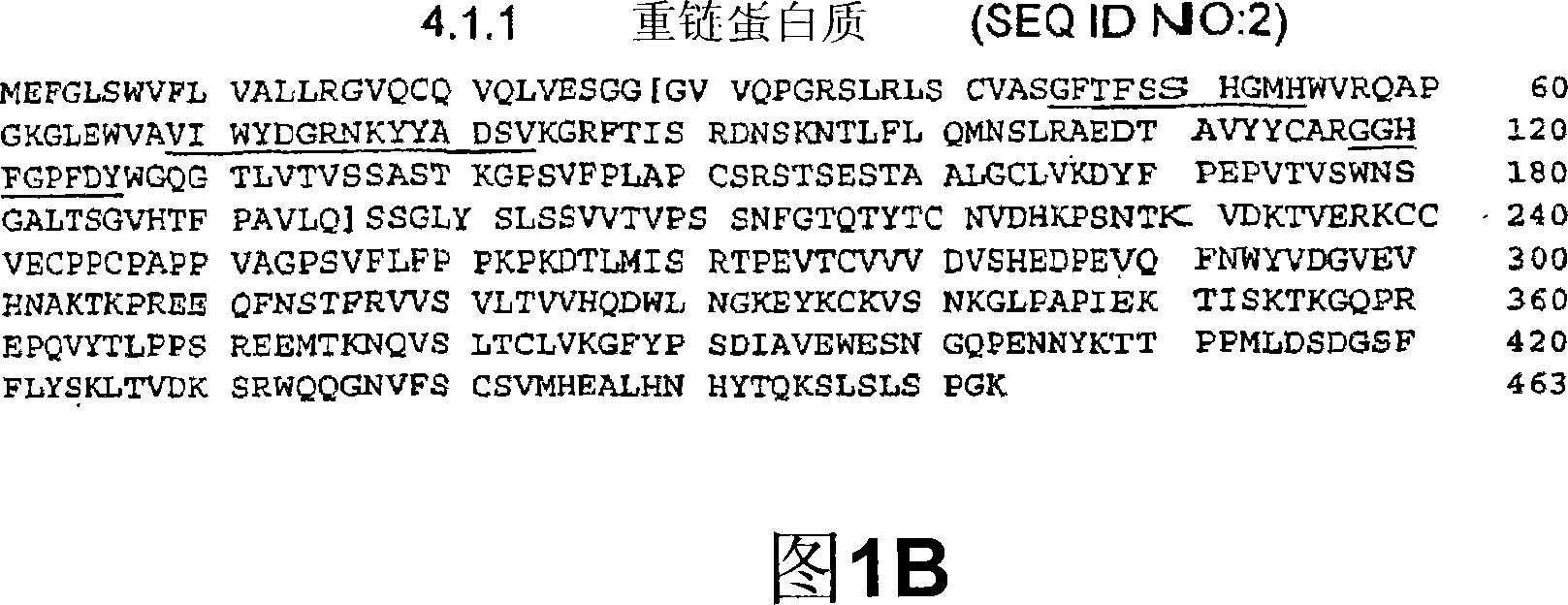
CTLA-4 antibody and aromatase inhibitor combination of treatment for breast cancer
An aromatase inhibitor, -CTLA4 technology, applied in the field of cancer immunotherapy, can solve the problems of patients with multiple diseases without radical therapy and palliative treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0230] The formulations of the pharmaceutical compositions described herein can be prepared by methods known or later developed in the art of pharmacy. In general, such methods of preparation comprise the steps of incorporating the active ingredient into the carrier or one or more other accessory ingredients, and then, if necessary or desired, shaping or packaging the product into desired single or multi-dose units.
[0231] The pharmaceutical compositions useful in the methods of the invention may be prepared, packaged, or administered in dosage forms suitable for oral, rectal, vaginal, parenteral, topical, pulmonary, intranasal, buccal, ophthalmic, or other routes of administration. Sale. Other contemplated formulations include contemplated nanoparticle, liposomal formulations, resealed red blood cells containing active ingredients, and immunological formulations.
[0232] The pharmaceutical compositions of the invention may be prepared, packaged or sold as a single unit do...
Embodiment
[0282] Anti-CTLA4 antibody combined with exemestane in postmenopausal patients with metastatic or locally advanced breast cancer
[0283] Intravenous infusion of anti-CTLA4 antibody (100ml / hr) was administered to patients with metastatic or locally advanced breast cancer, as described here, who had at least one lesion capable of accurate two-dimensional measurement and conventional CT scan Its size is ≥2cm×1cm, or its size is ≥1cm×1cm by spiral CT scan. Administer prophylactic antiemetics and antidiarrheals as appropriate.
[0284] The treatment was repeated after 28 days. Successive cohorts received escalating doses of anti-CTLA4 antibody, starting at 3 mg / kg and increasing to 6 mg / kg, 10 mg / kg, and 15 mg / kg after every 28 days, in the absence of intolerable toxicity or disease progression case for up to 12 consecutive cycles.
[0285] Preferably, the patient is given a premedication of antihistamine (H1 ) at least one and a half hours before anti-CTLA4 infusion. Premedic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com