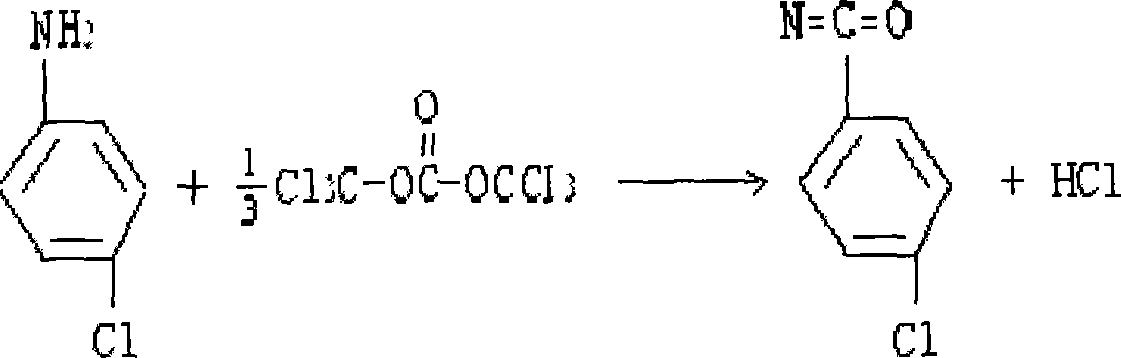Method of preparing 1-(4-chloro-phenyl)-3-(3,4-dichloro-phenyl)-urea
A technology of trichlorophenylene urea and p-chloroaniline, which is applied in 3 fields, can solve the problems of many impurities in the solvent, waste, and difficult disposal, and achieve the effects of high product yield, small environmental impact, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] ① Place a stirrer, a thermometer, and a condensing reflux device in a 1000ml reactor, add 100g of solid phosgene with a content ≥ 99%, and 300ml of toluene. The initial temperature of the addition is 35°C, and the temperature range is controlled below 60°C during the dropping process. After the dropwise addition, the temperature is kept for half an hour, then the temperature is raised to 80°C, and the temperature is kept for two hours. gas and the hydrogen chloride gas produced; when the material becomes clear, the reflux is stopped, and part of the toluene is evaporated; 40% p-chlorophenyl isocyanate solution is obtained, and solid impurities such as activated carbon are removed by filtration, and the yield is more than 87%;
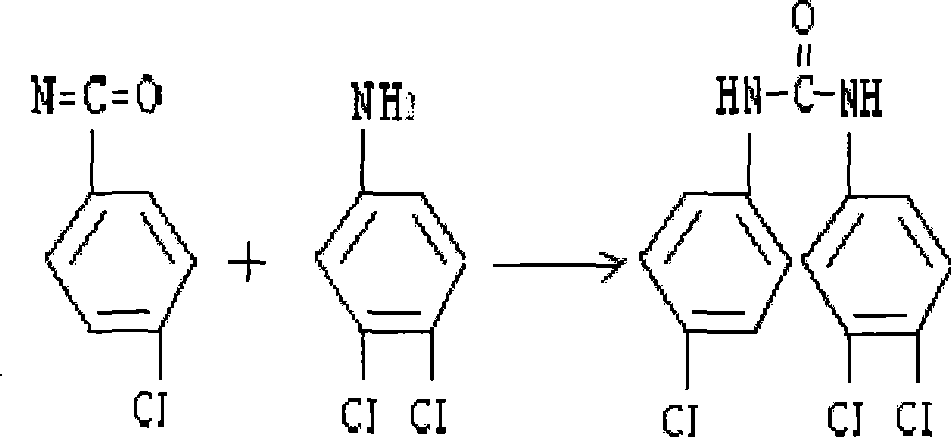
[0017] ②. In a 3000ml reactor, set a stirrer and a thermometer, and the reactor has a vent; add 105g of 3,4-dichloroaniline and 875ml of toluene to the reactor, and after heating, stirring and dissolving, add 40 % p-chlorophenyl isocyanate soluti...
Embodiment 2
[0020] 1. in embodiment 1, change solid phosgene and toluene consumption, solid phosgene increases to 105g, and toluene increases to 860ml, wherein the toluene consumption that is used to prepare p-chloroaniline is constant, and all the other repeat embodiment 1.
Embodiment 3
[0022] In 1. in embodiment 1, change solid phosgene and toluene consumption, solid phosgene is reduced to 82g, and toluene is reduced to 625ml, wherein the toluene consumption that is used to prepare p-chloroaniline is constant, all the other repeat embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com