Preparation process of plant estrin-enterodiol
A technology of phytoestrogens and enteric diols, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of difficulty in obtaining enteric diols and low yields, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
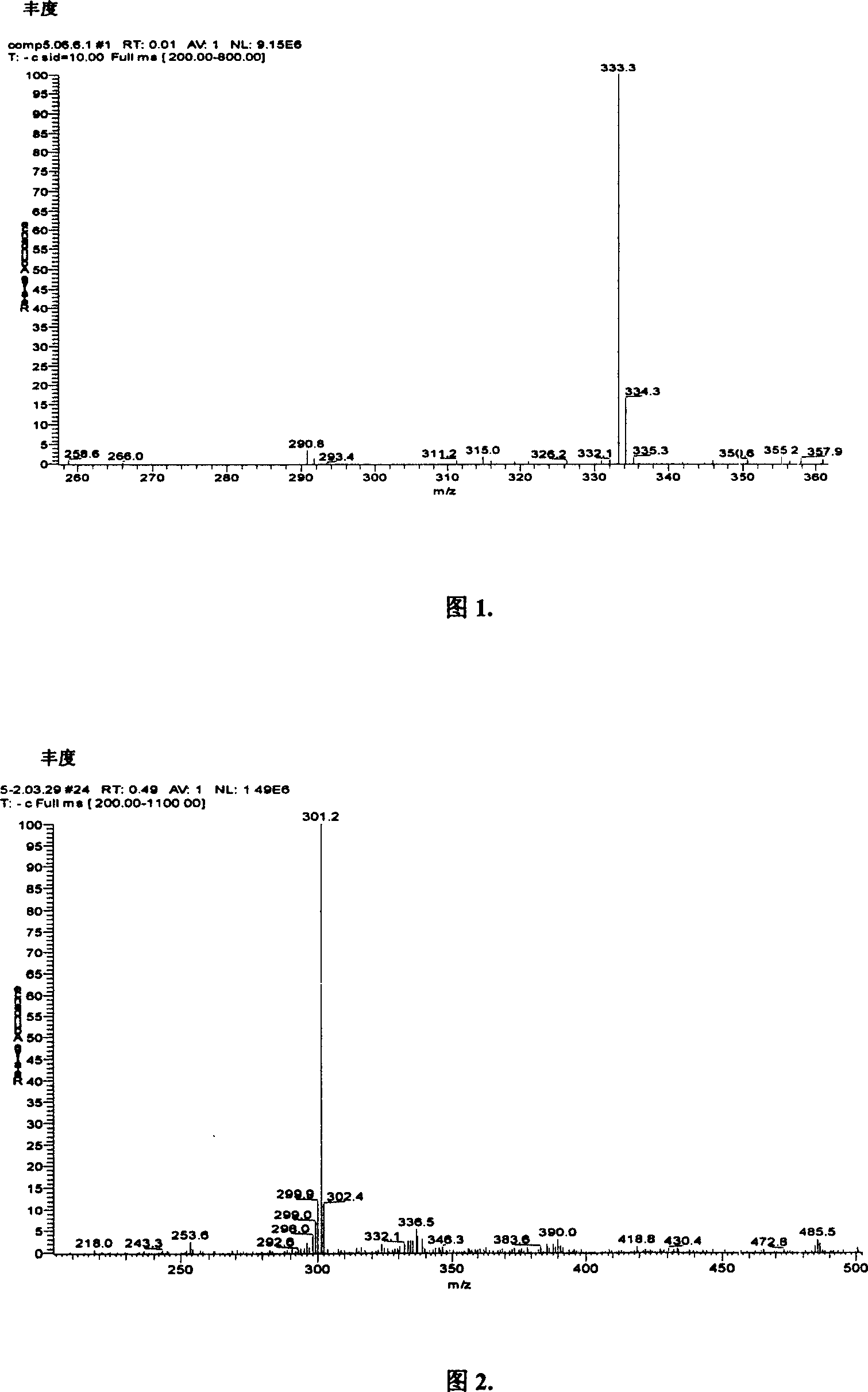
Image
Examples
Embodiment 1
[0025] (1), strain cultivation:
[0026] Add 1mg of 4,4'-dihydroxyenterodiol to 100mL of GAM broth medium, then add 0.1g of fresh feces from healthy people, and culture anaerobically at 35°C for 24 hours to obtain a bacterial culture solution; use this bacterial culture solution as a strain .
[0027] (2), prepare a kind of phytoestrogen-enterodiol:
[0028] "Add 30mg of 4,4'-dihydroxyenterodiol to 900mL of GAM broth medium, then add 100mL of the strain prepared according to the above step (1), and culture it anaerobically at 35°C for 1 day to obtain a culture solution. Use a concentration of 0.1 % (v / v) acetic acid water-saturated n-butanol extraction culture solution, the volume ratio of acetic acid water-saturated n-butanol solution to the strain culture solution is 1: 1. The extract is concentrated under reduced pressure, separated and purified by a silica gel column to obtain 20mg A phytoestrogen - enterodiol.
Embodiment 2
[0030] (1), strain cultivation:
[0031] Add 100mg of 4,4'-dihydroxyenterodiol to 600mL of GAM broth medium, then add 6.0g of fresh feces from healthy people, and incubate anaerobically at 37°C for 36 hours to obtain a bacterial culture solution; use this bacterial culture solution as a strain .
[0032] (2), prepare a kind of phytoestrogen-enterodiol:
[0033] Add 700 mg of 4,4'-dihydroxyenterodiol to 400 mL of GAM broth medium, then add 600 mL of the strain prepared according to the above step (1), and culture anaerobically at 37° C. for 5 days. The culture solution was extracted with 0.1% (v / v) acetic acid water-saturated n-butanol, and the volume ratio of the acetic acid water-saturated n-butanol solution to the culture solution was 1:1. The extract was concentrated under reduced pressure, separated and purified through a silica gel column to obtain 400 mg of a phytoestrogen-enterediol.
Embodiment 3
[0035] (1), strain cultivation:
[0036] Add 2000mg of 4,4'-dihydroxyenterodiol to 800mL of GAM broth medium, then add 160g of fresh feces from healthy people, and culture it anaerobically at 40°C for 48 hours to obtain a bacterial culture solution; this bacterial culture solution is used as the strain.
[0037] (2), prepare a kind of phytoestrogen-enterodiol:
[0038] Add 3000mg of 4,4'-dihydroxyenterodiol to 200mL of GAM broth medium, then add 800mL of the strain prepared in the above step (1), and culture anaerobically at 35-40°C for 10 days. The culture solution was extracted with 0.1% (v / v) acetic acid water-saturated n-butanol, and the volume ratio of the acetic acid water-saturated n-butanol solution to the culture solution was 1:1. The extract was concentrated under reduced pressure, separated and purified through a silica gel column to obtain 1500 mg of a phytoestrogen-enterediol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com