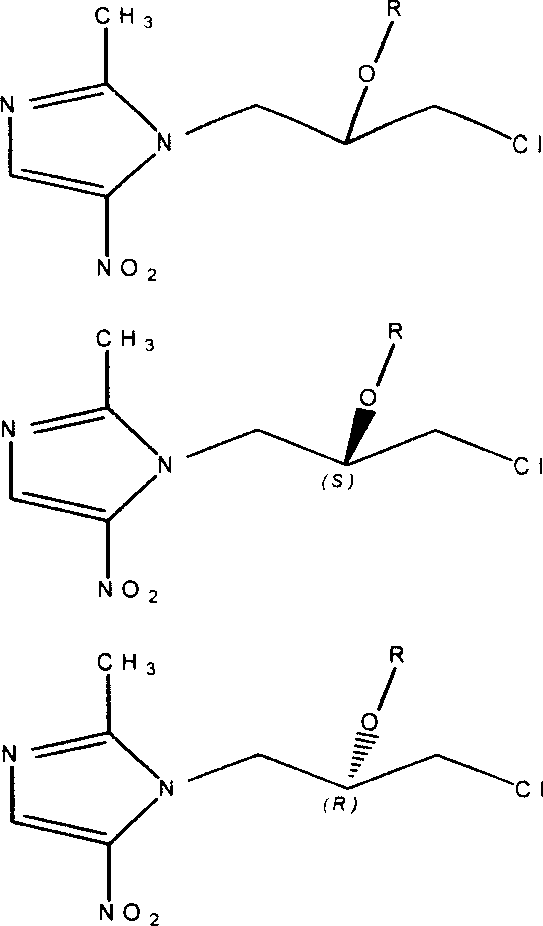
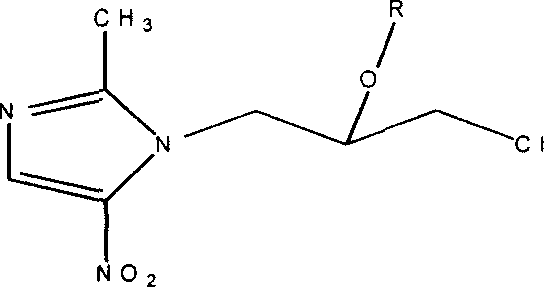
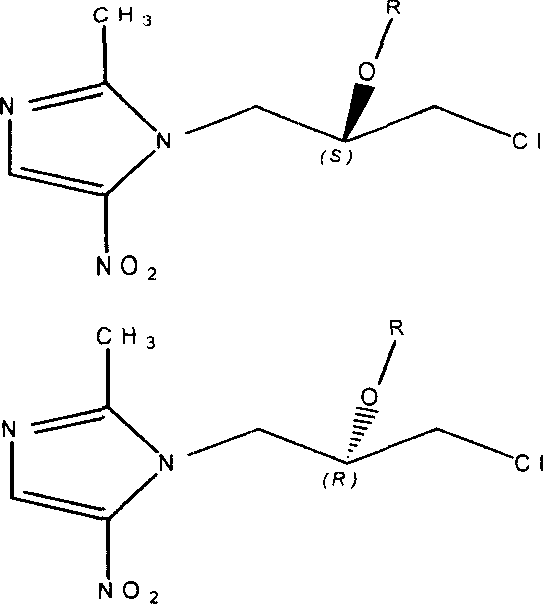
Onitrodazole precursor drug and its preparation process and use
A technology of prodrug and ornidazole, applied in the field of prodrug of ornidazole and its preparation and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of L-ornidazole acetate
[0036] Dissolve 110g of L-ornidazole in 500ml of dry ethyl acetate solution, add 90ml of acetyl chloride dropwise, control the reaction temperature at 10-20°C, control the reaction in the liquid phase until there is almost no peak of L-ornidazole, and recover acetic acid under reduced pressure Ethyl ester, under cooling, slowly add 600ml of pure water, slowly add 10% sodium carbonate solution to adjust the pH value to 5.0, concentrate to dryness under reduced pressure, add 500ml of ethanol, filter, add 500ml of petroleum ether to the filtrate, freeze and crystallize, Filter to obtain 91 g of L-ornidazole acetate.
[0037] [purity: 98.8%, chromatographic conditions: mobile phase is methanol: potassium dihydrogen phosphate solution of 0.05mol / L (adjusting pH with triethylamine is 6.0)=35: 65; Chromatographic column: Kromasil C18 5u, 250 * 4.6mm ;]
Embodiment 2
[0038] Embodiment 2: Preparation of L-ornidazole propionate
[0039] Dissolve 110g of L-ornidazole in 500ml of dry ethyl acetate solution, add 100ml of propionyl chloride dropwise, control the reaction temperature at 10-20°C, control the reaction in the liquid phase until there is almost no peak of L-ornidazole, and recover acetic acid under reduced pressure Ethyl ester, under cooling, slowly add 600ml of pure water, slowly add 10% sodium carbonate solution to adjust the pH value to 5.0, concentrate to dryness under reduced pressure, add 500ml of ethanol, filter, add 500ml of petroleum ether to the filtrate, freeze and crystallize, After filtration, 97 g of levo-ornidazole propionate was obtained.
[0040] [Purity: 99.1%, chromatographic conditions: mobile phase is methanol: potassium dihydrogen phosphate solution of 0.05mol / L (adjusting pH with triethylamine is 6.0)=35: 65; Chromatographic column: Kromasil C18 5u, 250 * 4.6mm ;]
Embodiment 3
[0041] Embodiment 3: the preparation of L-ornidazole benzoate
[0042] Dissolve 110g of L-ornidazole in 500ml of dry ethyl acetate solution, add 120ml of benzoyl chloride dropwise, control the reaction temperature at 10-20°C, control the reaction in the liquid phase until there is almost no peak of L-ornidazole, and recover under reduced pressure Ethyl acetate, under cooling, slowly add 600ml of pure water, slowly add 10% sodium carbonate solution to adjust the pH value to 5.0, concentrate to dryness under reduced pressure, add 500ml of ethanol, filter, add 500ml of petroleum ether to the filtrate, freeze and crystallize , filtered to obtain L-ornidazole benzoate 109g.
[0043] [purity: 98.3%, chromatographic condition: mobile phase is methanol: 0.05mol / L potassium dihydrogen phosphate solution (adjusting pH with triethylamine is 6.0)=35: 65; Chromatographic column: Kromasil C18 5u, 250 * 4.6mm ;]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com