Chitosan thiosemicarbazone derivatives and preparation method thereof
A technology of thiosemicarbazone and derivatives, which is applied in the field of chitosan thiosemicarbazone derivatives and the preparation thereof, can solve problems such as poor effect, achieve good water solubility, overcome poor solubility, and be easily absorbed. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 0.612g of acetylated chitosan with a molecular weight of 08,000 was dissolved in 80ml of 2% HAc, and 1.19g of thioanisemicarbazide was added under stirring, and reacted at 100°C for 8 hours. Concentrate under reduced pressure and cool to room temperature. Pour the reaction mixture into 300ml of absolute ethanol to obtain a precipitate. After standing for 8 hours, filter and wash the precipitate with absolute ethanol, dry at 50°C to obtain a brown powder, and use absolute ethanol as a solvent for Soxhlet extraction. After drying for 1 hour, the thiosemicarbazone derivative of acetylated chitosan is obtained. The structural formula is shown in formula 1, wherein R=-CH 3 , R 1 for n=49.
[0030] For the preparation of the acetylated chitosan, refer to Huang Liyao, Liu Chao, Preparation of Hydrophobic Long Fatty Chain Acylated Chitosan, Journal of Huaqiao University, 439-441.
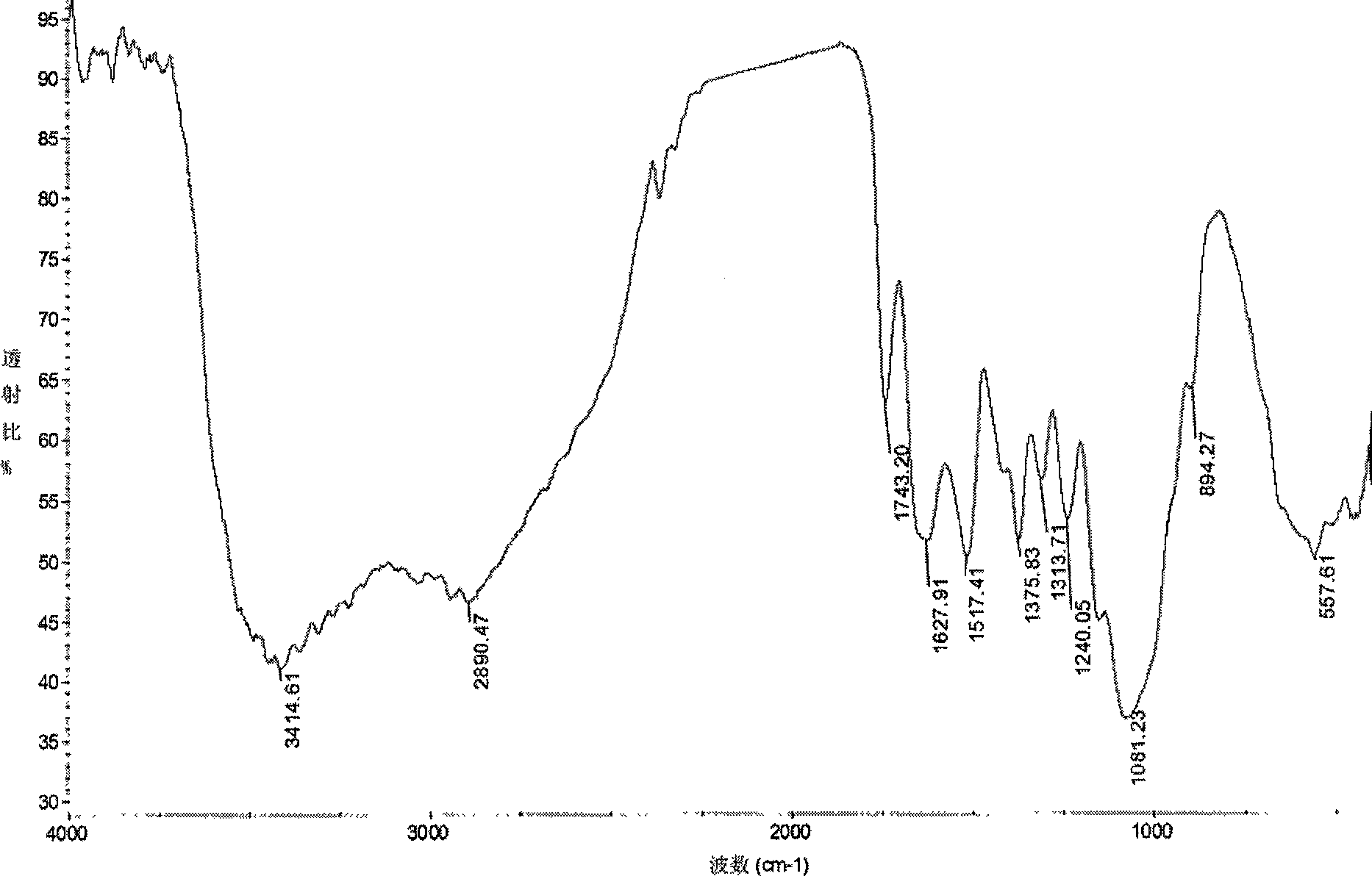
[0031] Infrared spectroscopy analysis shows that the acetylthiosemicarbazone derivatives of c...
Embodiment 2
[0033] 0.90 g of acetylated chitosan with a molecular weight of 200,000 was dissolved in 80 ml of 2% HAc, and 1.0 g of thioanisemicarbazide was added under stirring, and reacted at 100° C. for 10 hours. Concentrate under reduced pressure and cool to room temperature, pour the reaction mixture into 300ml of absolute ethanol to obtain a precipitate, filter and wash the precipitate with absolute ethanol, dry at 55°C to obtain a brown powder, use absolute ethanol as a solvent for Soxhlet extraction for 10 hours, and dry Obtain the thiosemicarbazone derivative of acetylated chitosan, the structural formula is referring to formula 1, wherein R=-CH 3 , R 1 for n=1240.
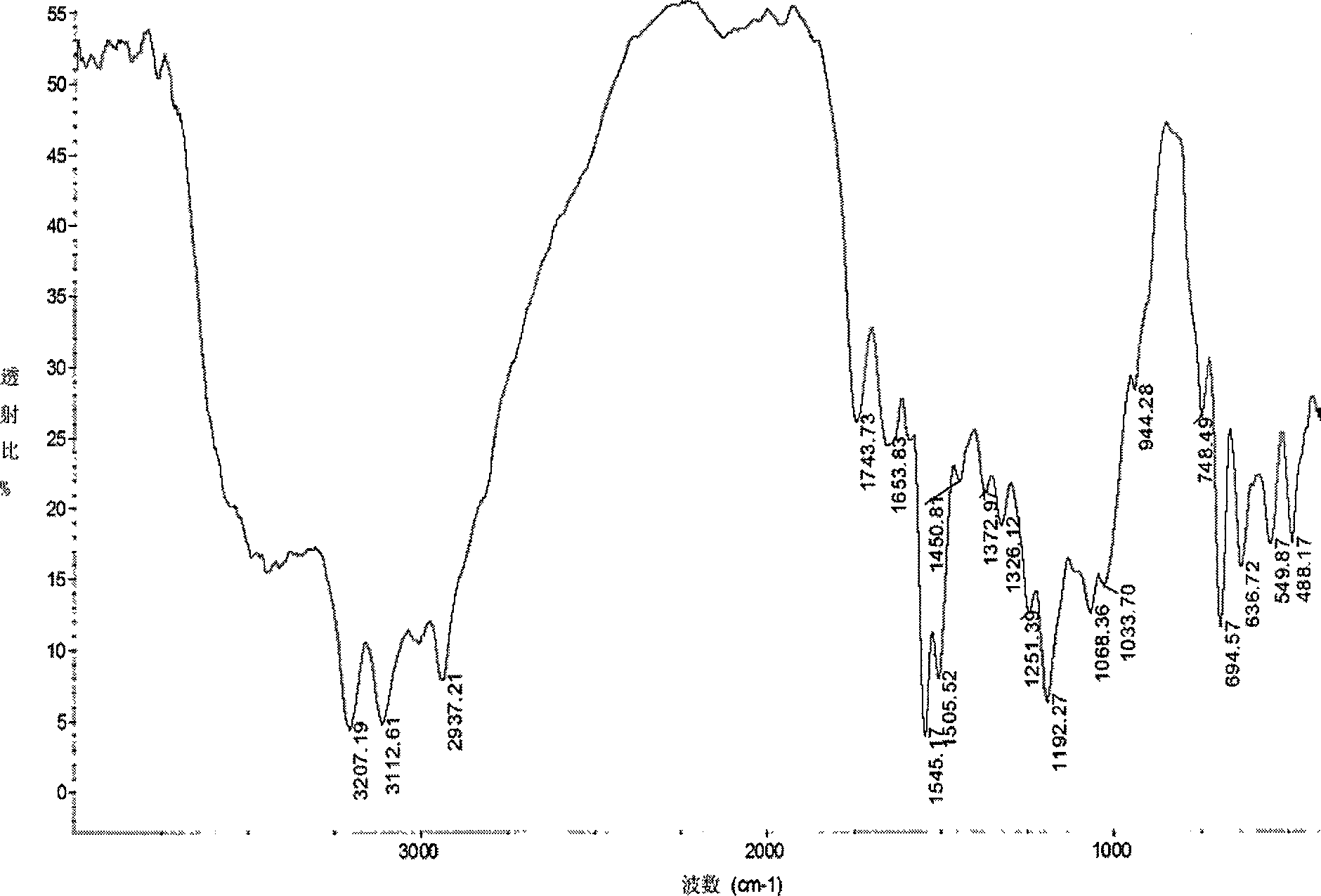
[0034] Infrared spectroscopy analysis shows that the acetylthiosemicarbazone derivatives of chitosan (see image 3 ) and acetylated chitosan (see figure 1 ) compared to: at 3207 and 3112cm -1 The sharper absorption peak at v NH The characteristic absorption peak of 1653.82cm -1 It is the characteristic absorpt...
Embodiment 3
[0036] 0.738g of acetylated chitosan with a molecular weight of 20,000 was dissolved in 60ml of 2% HAc, and 1.086g of p-toluenethiosemicarbazide was added under stirring, and reacted at 100°C for 9 hours. Concentrate under reduced pressure and cool to room temperature. Pour the reaction mixture into 300ml of acetone to obtain a precipitate. After filtering, wash the precipitate with acetone and dry at 50°C to obtain a brown powder. Soxhlet extract with absolute ethanol for 8 hours and then dry to obtain the acetylated shell. p-toluidine thiosemicarbazone derivatives of polysaccharides, the structural formula is shown in formula 1, wherein R=-CH 3 , R 1 for n=124.
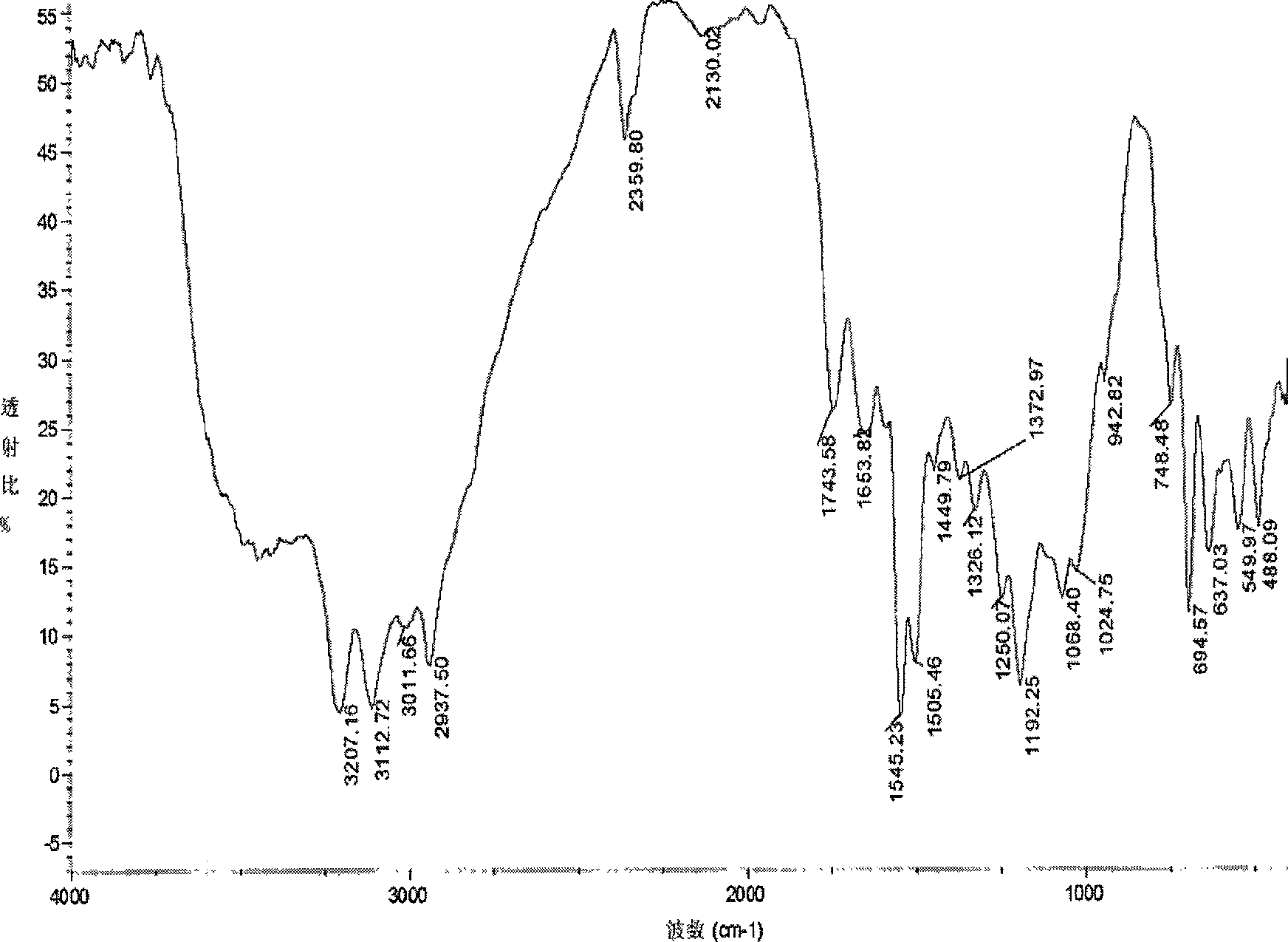
[0037] Infrared spectroscopy analysis shows that the acetyl p-toluidine thiosemicarbazone derivatives of chitosan (see Figure 4 ) and acetylated chitosan (see figure 1 ) compared to: at 3193 and 3120cm -1 The sharper absorption peak at v NH The characteristic absorption peak, 1660cm -1 It is the characteris...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com