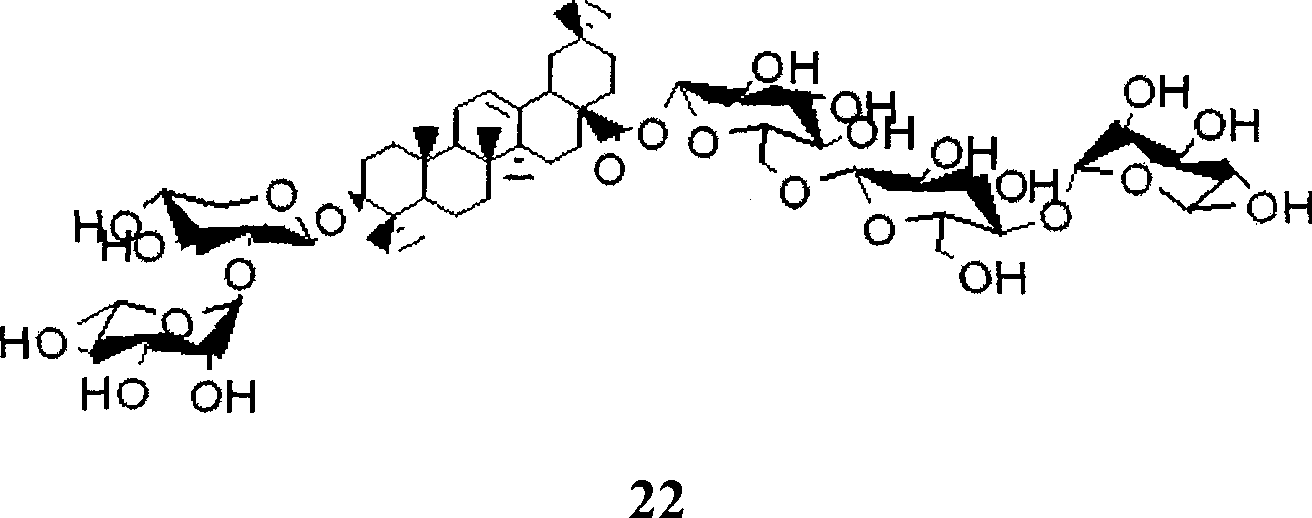
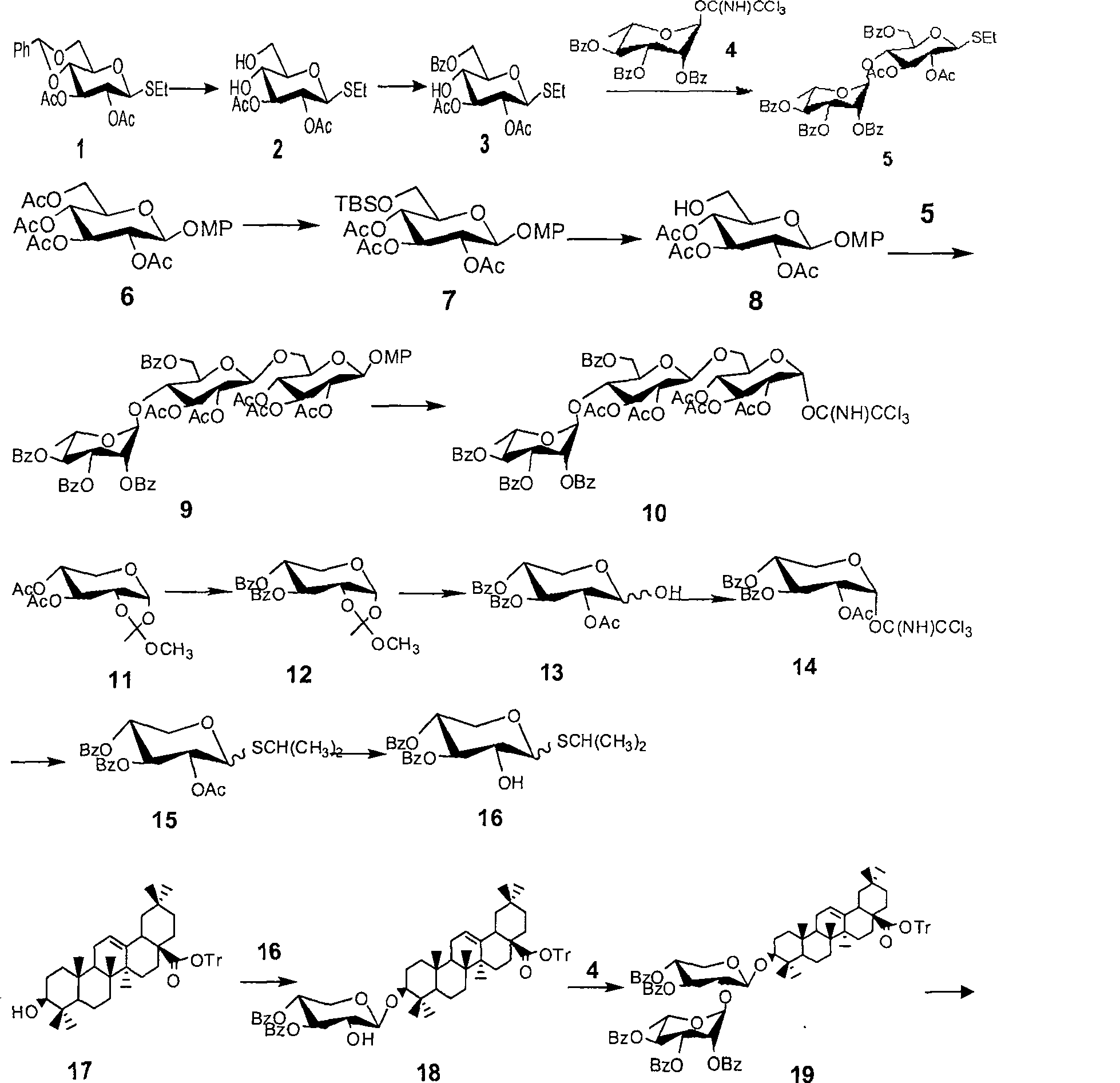
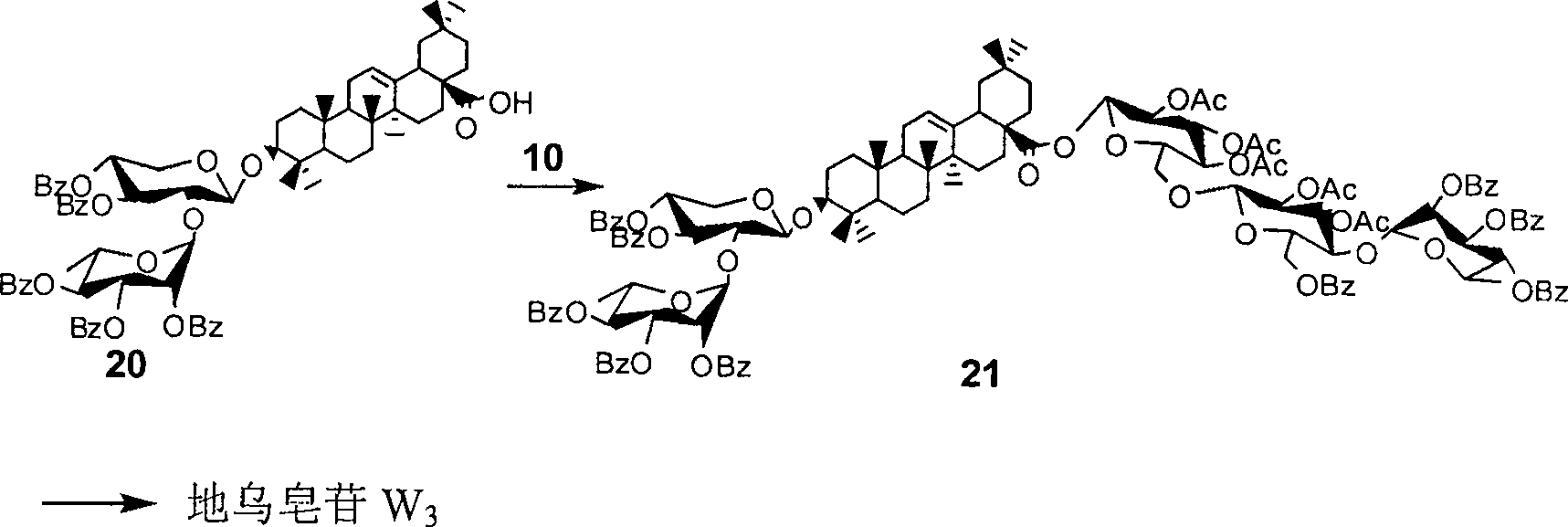
Method for synthesizing anemone flaccida saponins W3
A synthesis method and technology of wuli saponin are applied in the field of chemical synthesis of the active component of traditional Chinese medicine diguanoside W3 to achieve the effects of simple method, solving the shortage of plant resources and not being limited by resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of embodiment 1 compound 5
[0036] Compound 1 (10.0 g, 25.2 mmol) was dissolved in 100 ml of acetic acid aqueous solution, heated to reflux for 4 to 6 hours, concentrated under reduced pressure, purified and separated by column chromatography to obtain compound 2 (7.38 g, 95%); In 42 ml of pyridine, stir under an ice-water bath, dissolve benzoyl chloride (BzCl, 3.1 ml, 26.3 mmol) in 10 ml of pyridine, drop into the solution of 2 above, remove the ice-water bath, and react at room temperature for 3-8 hours, concentrated under reduced pressure, separated by column chromatography to obtain compound 3 (8.88 g, 90%); under nitrogen protection, compound 3 (610 mg, 1.48 mmol) and 4 (1.10 g, 1.77 mmol) were dissolved in 12 ml Dichloromethane, stirred at 0°C, and added trimethylsilyl trifluoromethanesulfonate (TMSOTf), reacted for 10 to 50 minutes, TLC detection showed that the reaction was complete, added alkali to neutralize the reaction system, concentrated und...
Embodiment 2
[0037] The synthesis of embodiment 2 compound 10
[0038] Dissolve compound 6 (8.0 g, 17.6 mmol) in 80 ml of methanol, stir at room temperature, add sodium methoxide (NaOMe) dropwise to make the pH value between 9 and 11, react at room temperature, and detect the reaction by thin layer chromatography After completion, neutralize the reaction solution with an acidic resin to neutrality, filter off the resin, concentrate the filtrate, drain the solvent under reduced pressure, dissolve it in 50 ml of pyridine, add tert-butyldimethylsilyl chloride (TBSCl), and stir at room temperature for 2- 5 hours, then add acetic anhydride (Ac 2 (0), concentrated under reduced pressure after 2 to 5 hours, and purified and separated by column chromatography to obtain a white solid 7 (8.81 grams, 95%); 7 was dissolved in 80 milliliters of dichloromethane, and boron trifluoride ether (BF 3 ·Et 2 O), stirring at room temperature for 30-50 minutes, thin-layer chromatography detected that the react...
Embodiment 3
[0039] The synthesis of embodiment 3 compound 22
[0040] Dissolve compound 11 (10.0 g, 34.5 mmol) in 80 ml of methanol, stir at room temperature, add sodium methoxide in methanol dropwise to keep the pH between 9 and 11, react at room temperature for 2 to 4 hours, and thin layer After the chromatographic detection reaction was complete, it was concentrated, purified and separated by column chromatography, and the oil was dissolved in 40 milliliters of pyridine, stirred in an ice-water bath, benzoyl chloride (BzCl) was dissolved in 15 milliliters of pyridine, dropped into the above solution, and stirred at room temperature for 3 ~6 hours, TLC detection showed that the reaction was complete, concentrated under reduced pressure, purified and separated by column chromatography to obtain compound 12 (12.8 grams, 90%); 12 (12.0 grams, 30.0 mmol) was dissolved in 120 milliliters of acetic acid solution, 30 After stirring for 3-6 hours at ~40°C, it was concentrated under reduced pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com