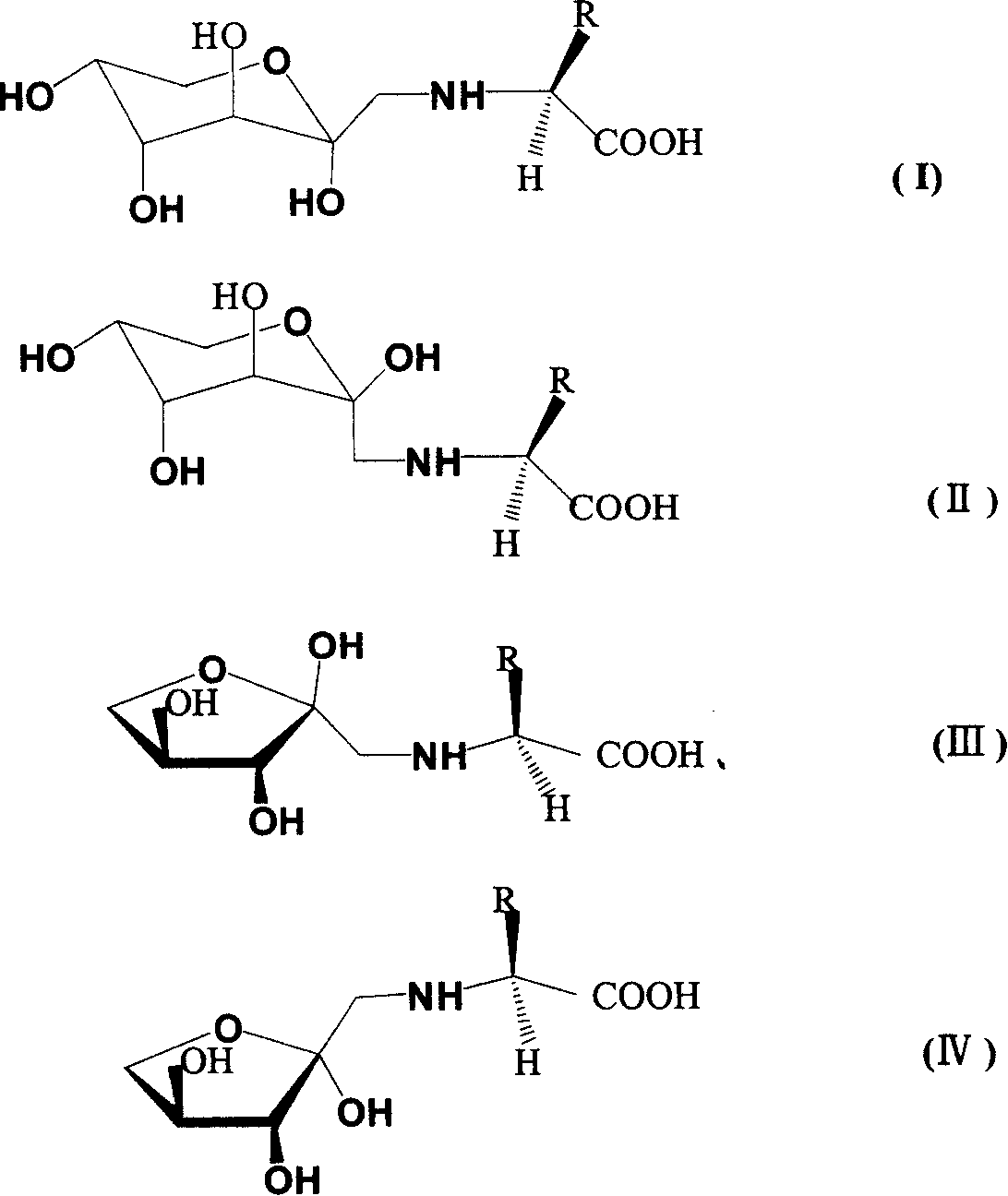
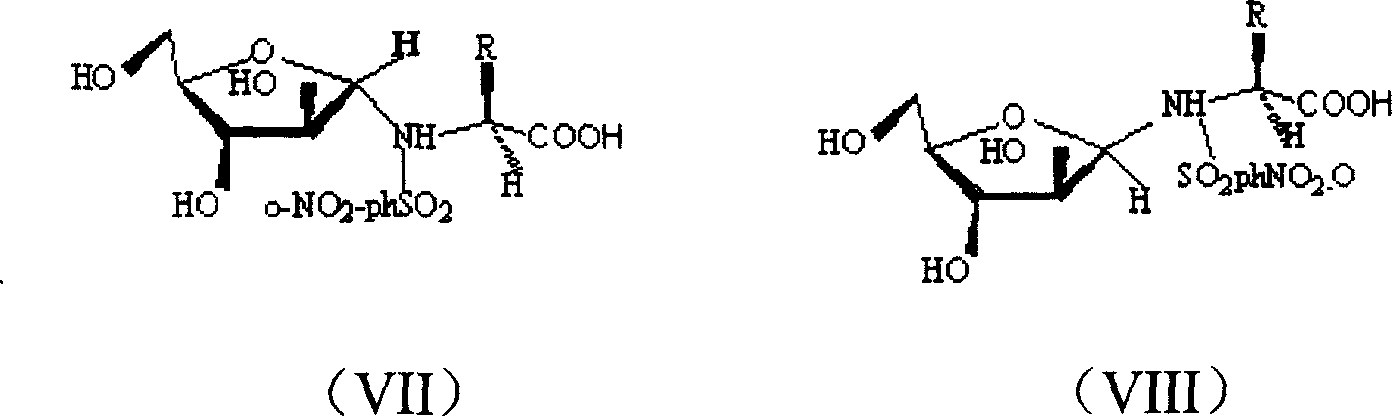
Cyclo-glycosyl amino acid, its preparation method and usage in preventing and curing heavy metal poisoning
An amino acid and heavy metal technology, applied in acyclic sugars, antidote, drug combination, etc., can solve the problems of poor cell membrane selectivity, difficult to cross, and large toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1L-aspartic acid di-tert-butyl ester
[0063] A mixture of 3.99g (30mmol) L-aspartic acid, 412ml (66mmol) perchloric acid and 168ml tert-butyl acetate was stirred at room temperature for 48 hours and placed in the refrigerator overnight. The reaction mixture was washed (4×100ml) with dilute hydrochloric acid (0.5mol / l). Separate the aqueous layer with solid NaHCO 3 Neutralized to PH7-8, extracted with ethyl acetate (8×50ml), the combined ethyl acetate layer was washed with saturated NaHCO 3 Wash with aqueous solution (2×200ml), anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to obtain 6.4 g (76%) of the hydrochloride salt of the title compound as a pale yellow solid. Rf (petroleum ether / ethyl acetate, 2:1) was 0.26. The product was directly used in the next reaction without purification.
Embodiment 2
[0064] The preparation of embodiment 2L-S-tert-butylcysteine tert-butyl ester
[0065] According to the operation of Example 1, 4.80 g (30 mmol) of L-S-tert-butylcysteine was used instead of L-aspartic acid to obtain 5.7 g (71%) of the title compound as a light yellow solid. Rf (petroleum ether / ethyl acetate, 2:1) was 0.3. The product was directly used in the next reaction without purification.
Embodiment 3
[0066]The preparation of embodiment 3L-di-tert-butyl glutamate
[0067] According to the operation of Example 1, 4.41 g (30 mmol) of L-glutamic acid was used instead of L-aspartic acid to obtain 6.2 g (80%) of the title compound as a light yellow solid. Rf (petroleum ether / ethyl acetate, 2:1) was 0.15. The product was directly used in the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com