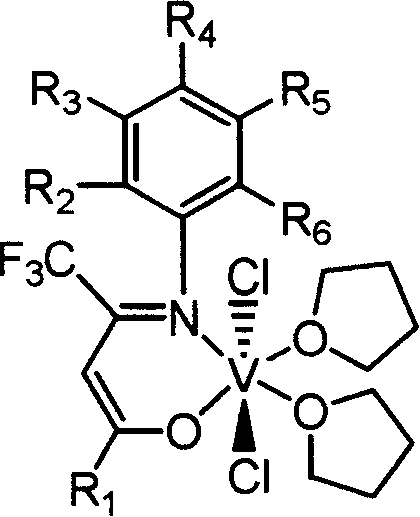
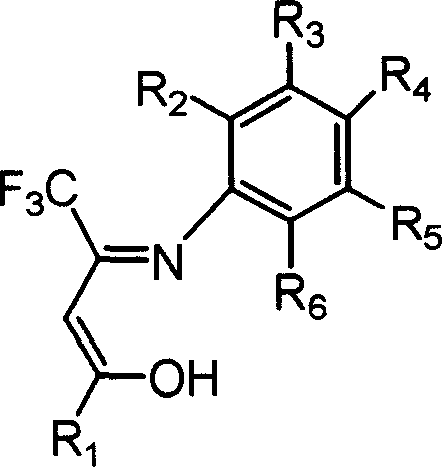
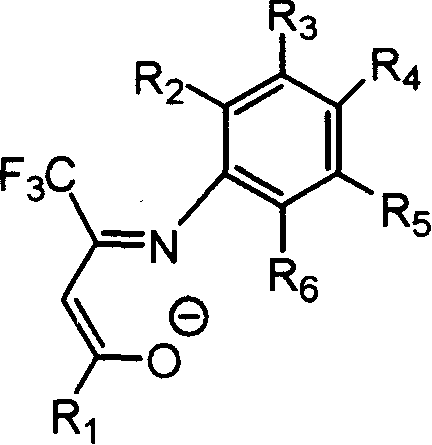
Beta-diketone monoimine vanadium catalyst containing trifluoromethyl radical for olefinic polymerization and its preparation method and uses
A technology of diketone monoimine vanadium olefin and polymerization catalyst, which is applied in the field of β-diketone monoimine vanadium olefin polymerization catalyst and its preparation, and can solve problems such as weak ability and poor high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 1,1,1-trifluoro-5,5-dimethyl-2,4-hexanedione 7.85g, equivalent to 40mmol, aniline 3.73g, equivalent to 40mmol, methanol 15mL, Formic acid 1mL, heated to reflux for 24h. The solvent methanol was distilled off with a rotary evaporator, and petroleum ether containing 1% ethyl acetate was used as eluent, and the residue was subjected to column chromatography to obtain 5.64 g of white solid Schiff's base, with a yield of 52%. According to mass spectrometry, the molecular ion peak m / e is 271. Elemental analysis found values: C, 61.78%; H, 5.90%; N, 5.19%; theoretical value (C14H16F3NO): C, 61.98%; H, 5.94%; N, 5.16%.
[0035] Under nitrogen protection, 0.54 g of the Schiffer base obtained in Example 1, equivalent to 2 mmol, and 20 mL of anhydrous tetrahydrofuran were added to a dry 100 mL reaction flask, stirred at room temperature for 10 min, and cooled to -78°C. Within 5 minutes, 1.375 mL of a 1.60 mol / L n-butyllithium hexane solution, equivalent to 2.2 mmol, was adde...
Embodiment 2
[0037] 4.29 g of o-methylaniline, equivalent to 40 mmol, was used to replace the aniline in Example 1, and the experimental operation was the same as in Example 1 to obtain 5.48 g of white solid Schiff's base, with a yield of 48%. According to mass spectrometry, the molecular ion peak m / e is 285. Elemental analysis found values: C, 63.32%; H, 6.31%; N, 4.94%; theoretical value (C15H18F3NO): C, 63.15%; H, 6.36%; N, 4.91%.
[0038] Using 0.57 g of Schiffer's base prepared in Example 2, which is equivalent to 2 mmol to replace the Schiffer's base obtained in Example 1, the experimental operation is the same as in Example 1, and brown β-diketone monoimine vanadium olefin containing trifluoromethyl is obtained The polymerization catalyst was 0.54 g, and the yield was 49%. According to mass spectrometry, the molecular ion peak m / e is 550. Elemental analysis found values: C, 50.10%; H, 5.99%; N, 2.53%; theoretical value (C22H33Cl2F3N03V): C, 50.19%; H, 6.04%; N, 2.55%.
Embodiment 3
[0040] 5.41 g of 2,4,6-trimethylaniline, equivalent to 40 mmol, was used to replace the aniline in Example 1, and the experimental operation was the same as in Example 1 to obtain 5.26 g of white solid Schiff's base, with a yield of 42%. According to mass spectrometry, the molecular ion peak m / e is 313. The measured value of elemental analysis; C, 65.29%; H, 7.12%; N, 7.42%; theoretical value (C17H22F3NO): C, 65.16%; H, 7.08%; N, 4.47%.
[0041] Using 0.63 g of Schiffer's base prepared in Example 3, which is equivalent to 2 mmol to replace the Schiffer's base obtained in Example 1, the experimental operation is the same as in Example 1, to obtain brown β-diketone monoimine vanadium olefin containing trifluoromethyl The polymerization catalyst was 0.52 g, and the yield was 45%. According to mass spectrometry, the molecular ion peak m / e is 578. Elemental analysis found values: C, 51.72%; H, 6.48%; N, 2.39%; theoretical value (C25H37Cl2F3NO3V): C, 51.91%; H, 6.45%; N, 2.42%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com