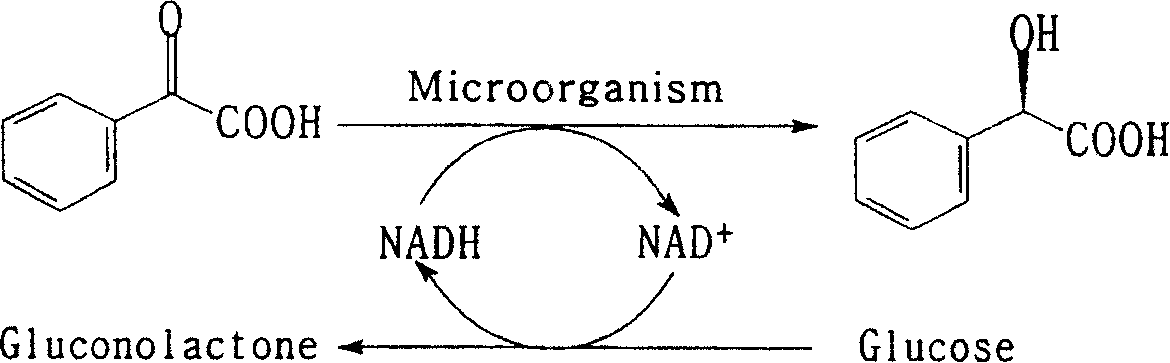
Process for preparing (R)-mandelic acid by microbial asymmetric reduction
A microbial method and asymmetric technology, applied in the field of biological asymmetric synthesis of chiral compounds, can solve problems such as difficult separation, difficult separation of product and substrate, and many by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Cultivation of bacterial strains: the composition of the medium is that the components contained in each 100ml of culture solution are in g: glucose 2-6, yeast extract 0.25-1, (NH 4 ) 2 HPO 4 0.5~2.0, KH 2 PO 4 0.1~0.5, Mg8O 4 ·7H 2 O 0.02~0.06, NaCl 0.0025~0.01, ZnSO 4 ·7H 2 O 0.001~0.005, FeSO 4 ·7H 2 O 0.001~0.005, CuSO 4 ·5H 2 O 0.0001~0.0005, MnSO 4 4H 2 O 0.00025~0.001.
[0061] The culture conditions are as follows: the initial pH is 6.0-8.0, the filling volume is 20%, the culture temperature is 30° C., the rotation speed of the shaking flask is 100-300 rpm, and the culture time is 48 hours.
Embodiment 2
[0063] Preparation of whole cells: Saccharomyces cerevisiae AS 2.150 was inoculated in a 250ml shake flask with a liquid volume of 20% and shaken at 30°C and 100-300rpm for 48 hours; After washing twice with saline, the collected cells were stored in a refrigerator at 4°C for transformation reactions.
Embodiment 3
[0065] Weigh 0.1g (5%) Saccharomyces cerevisiae AS 2.150 bacterial cells, place in 2mL potassium phosphate buffer containing 0.2mol / L, pH7.0 containing 5mg benzoylformic acid, add 0.01g glucose, 40 ℃, 150 rpm, transformed for 24 hours, centrifuged the transformed liquid, collected the supernatant, adjusted the pH to 1.0 with 3 mol / L hydrochloric acid, then extracted with 2.5 times the volume of ethyl acetate, and the separated ethyl acetate layer was washed with anhydrous Remove water with sodium sulfate, dry in vacuum at low temperature, dissolve the residual solid with a mixture of n-hexane and isopropanol (volume ratio 9:1), the product (R)-mandelic acid has an optical purity of 99.89% e.e, and a yield of 84.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com