Sustained release composition for oral administration of drugs
A slow-release composition, the technology of the composition, which is applied in the directions of drug combination, drug delivery, antipyretic drug, etc., can solve problems such as easy degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-21
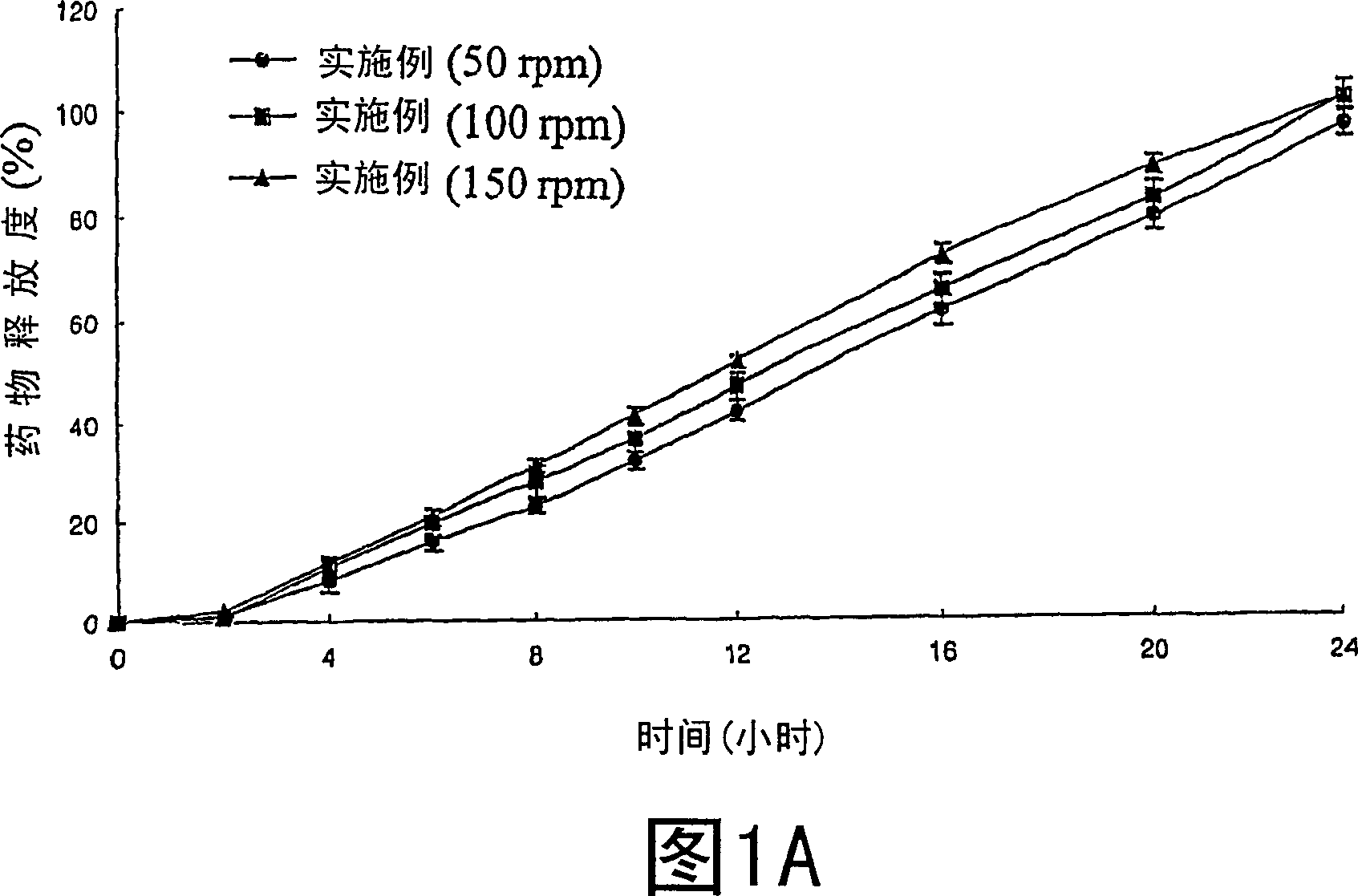
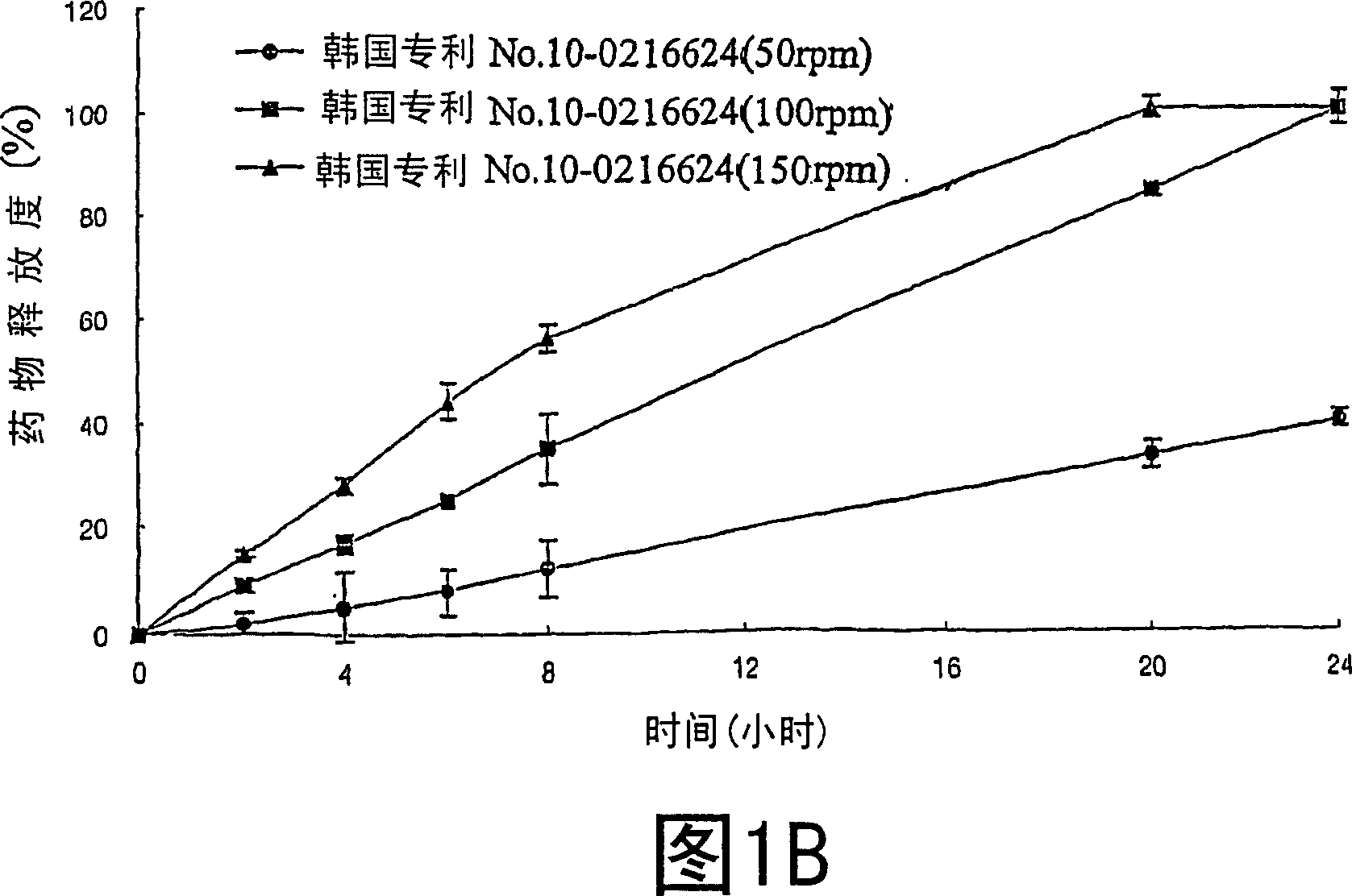
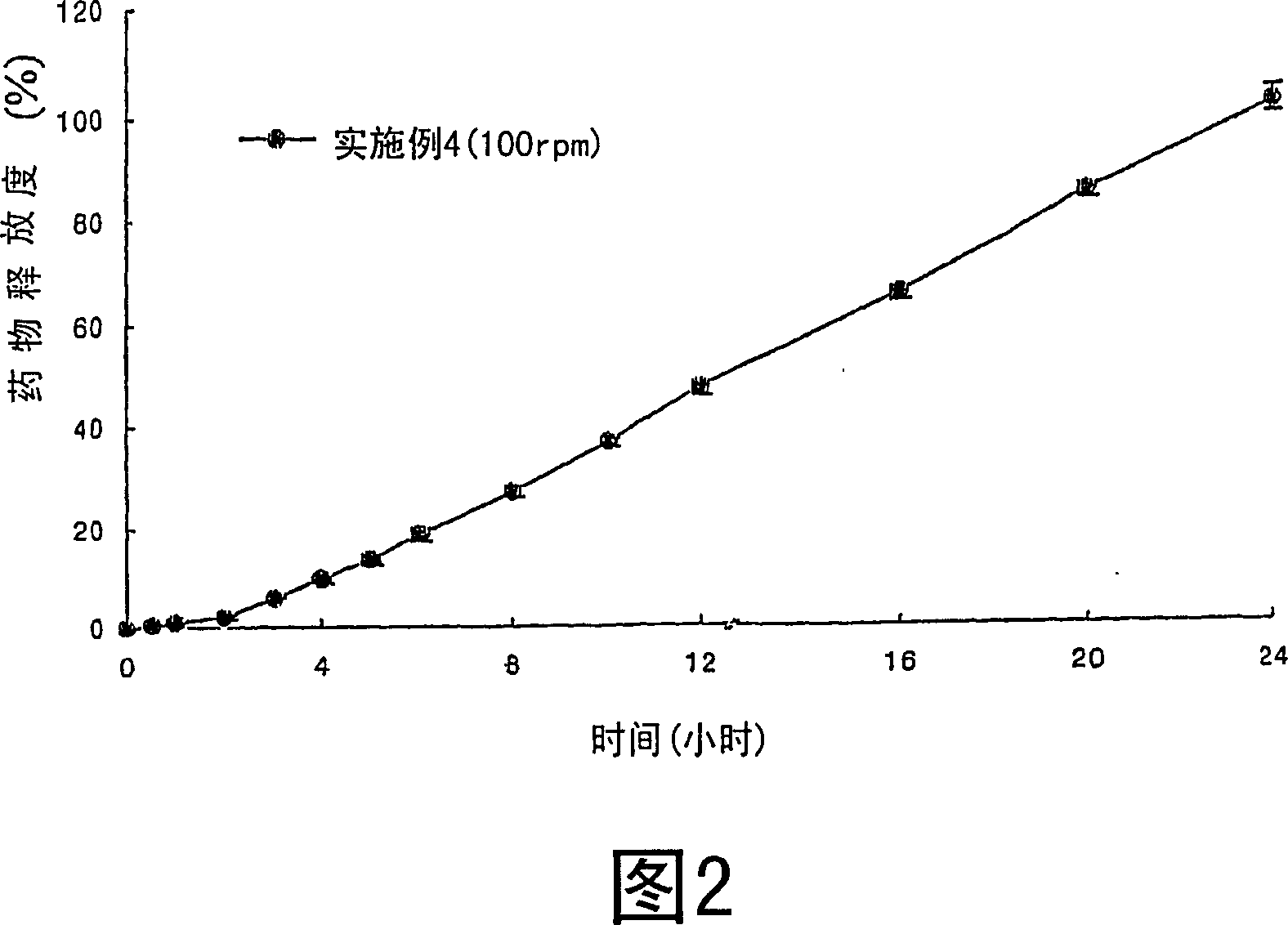
[0035] Examples 1-21: Preparation of Tablets
[0036] Nifedipine (Unique Chemicals, India), Isradipine (Sun Pharm.India), Lovastatin (CKD Pharm.KOREA), Glipizide (Sun Pharm.India), Sodium Alginate (Keltone HVCR, Keltoen LVF, Kelcosol , Kelset , ISP USA), xanthan gum (Keltrol F, Kelco, USA), locust bean gum (Cesagum LN1, LR200, Cesalpinia, Italy), propylene glycol alginate (Kelcoloid HVF, LVF, ISP, USA), hydroxypropyl methylcellulose (Meltose 90SH, 4,000SR, 100,000SR, Shin-Etsu, Japan) and kofovidone ( Kollidon VA65, BASF, Germany) was mixed for 30 minutes as described in Table 1, magnesium stearate and light anhydrous silicic acid (below 30 mesh) were added thereto and mixed for 5 minutes. Then, the resulting mixture is tableted by a pressure commonly used in tablet preparation, thereby obtaining tablets.
[0037] drug
[0038] drug
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com