Role of Ionic Liquid Electrolytes in Potassium-Sulfur Batteries
OCT 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquid Electrolytes in K-S Batteries: Background and Objectives
Potassium-sulfur (K-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical high energy density, cost-effectiveness, and environmental sustainability. The evolution of this technology can be traced back to the broader development of alkali metal-sulfur battery systems, which began with lithium-sulfur batteries in the 1960s. Over the decades, research has expanded to include sodium and potassium counterparts as alternatives to address resource scarcity and cost concerns associated with lithium.
The technical trajectory of K-S batteries has been marked by significant challenges, particularly related to the shuttle effect of polysulfides, poor sulfur utilization, and rapid capacity fading. These issues have historically limited the practical application of K-S batteries despite their theoretical advantages. The introduction of ionic liquid electrolytes represents a critical turning point in addressing these fundamental challenges.
Ionic liquids, defined as salts with melting points below 100°C, offer unique properties including negligible vapor pressure, non-flammability, high thermal stability, and wide electrochemical windows. Their application in battery systems dates back to the early 2000s, but their specific implementation in K-S batteries has gained momentum only in the past decade as researchers seek alternatives to conventional organic electrolytes.
The technical objectives for ionic liquid electrolytes in K-S batteries are multifaceted. Primary goals include suppressing the potassium polysulfide shuttle effect, enhancing the stability of the potassium metal anode, improving the ionic conductivity at the electrode-electrolyte interface, and extending the cycle life of K-S batteries. Additionally, researchers aim to develop electrolyte formulations that can operate across wider temperature ranges while maintaining safety.
Recent technological trends indicate a shift toward task-specific ionic liquids designed explicitly for K-S battery applications. This includes the development of dual-function ionic liquids that can simultaneously serve as electrolytes and as components of the cathode structure to enhance sulfur utilization. Another emerging trend is the combination of ionic liquids with solid-state electrolyte concepts to create hybrid systems that leverage the advantages of both approaches.
The expected technical outcomes from this research direction include K-S batteries with energy densities approaching their theoretical maximum (1023 Wh/kg), coulombic efficiencies exceeding 99%, and cycle lives of over 1000 cycles with minimal capacity degradation. These advancements would position K-S batteries as viable alternatives to current lithium-ion technologies, particularly for grid-scale energy storage applications where cost and sustainability are paramount considerations.
The technical trajectory of K-S batteries has been marked by significant challenges, particularly related to the shuttle effect of polysulfides, poor sulfur utilization, and rapid capacity fading. These issues have historically limited the practical application of K-S batteries despite their theoretical advantages. The introduction of ionic liquid electrolytes represents a critical turning point in addressing these fundamental challenges.
Ionic liquids, defined as salts with melting points below 100°C, offer unique properties including negligible vapor pressure, non-flammability, high thermal stability, and wide electrochemical windows. Their application in battery systems dates back to the early 2000s, but their specific implementation in K-S batteries has gained momentum only in the past decade as researchers seek alternatives to conventional organic electrolytes.
The technical objectives for ionic liquid electrolytes in K-S batteries are multifaceted. Primary goals include suppressing the potassium polysulfide shuttle effect, enhancing the stability of the potassium metal anode, improving the ionic conductivity at the electrode-electrolyte interface, and extending the cycle life of K-S batteries. Additionally, researchers aim to develop electrolyte formulations that can operate across wider temperature ranges while maintaining safety.
Recent technological trends indicate a shift toward task-specific ionic liquids designed explicitly for K-S battery applications. This includes the development of dual-function ionic liquids that can simultaneously serve as electrolytes and as components of the cathode structure to enhance sulfur utilization. Another emerging trend is the combination of ionic liquids with solid-state electrolyte concepts to create hybrid systems that leverage the advantages of both approaches.
The expected technical outcomes from this research direction include K-S batteries with energy densities approaching their theoretical maximum (1023 Wh/kg), coulombic efficiencies exceeding 99%, and cycle lives of over 1000 cycles with minimal capacity degradation. These advancements would position K-S batteries as viable alternatives to current lithium-ion technologies, particularly for grid-scale energy storage applications where cost and sustainability are paramount considerations.
Market Analysis for Next-Generation Potassium-Sulfur Energy Storage
The global energy storage market is witnessing a significant shift towards more sustainable, efficient, and cost-effective solutions. Potassium-sulfur (K-S) batteries represent an emerging technology with substantial market potential, particularly as the limitations of lithium-ion batteries become increasingly apparent in grid-scale applications.
Current market projections indicate that the global grid energy storage market will reach approximately $15 billion by 2025, with an annual growth rate exceeding 20%. Within this expanding market, potassium-based technologies are positioned to capture a growing share due to their economic advantages over lithium-based alternatives.
The primary market drivers for K-S battery technology include the decreasing availability and increasing cost of lithium resources, coupled with the abundant nature of potassium as the seventh most common element in the Earth's crust. This abundance translates to significantly lower raw material costs, with potassium carbonate priced at roughly one-third the cost of lithium carbonate.
Market segmentation analysis reveals several key sectors poised for K-S battery adoption. The utility-scale energy storage sector represents the largest potential market, particularly in regions investing heavily in renewable energy integration. Commercial and industrial energy management systems form another substantial market segment, where cost-effectiveness is a critical factor in technology selection.
Regional market analysis shows varying adoption potential. Asia-Pacific, particularly China, leads in research investment and manufacturing capacity development for potassium-based energy storage. Europe follows with strong policy support for sustainable energy technologies, while North America shows growing interest driven by grid resilience concerns.
Consumer demand patterns indicate increasing preference for sustainable energy solutions with lower environmental impact. The reduced carbon footprint of K-S batteries compared to lithium-ion alternatives positions them favorably in environmentally conscious markets. Additionally, the non-flammable nature of certain ionic liquid electrolytes addresses safety concerns that have plagued other battery technologies.
Economic analysis reveals that K-S batteries with ionic liquid electrolytes could potentially achieve a levelized cost of storage below $150/kWh when manufactured at scale, representing a 30% reduction compared to current lithium-ion solutions for stationary applications.
Market barriers include technological maturity concerns, with investors cautious about committing to pre-commercial technologies. The established infrastructure for lithium-ion manufacturing also creates inertia against adoption of alternative chemistries. However, these barriers are gradually diminishing as demonstration projects prove the viability of K-S technology.
The competitive landscape includes both established battery manufacturers exploring potassium-based alternatives and startups focused exclusively on next-generation chemistries. Strategic partnerships between material suppliers, electrolyte developers, and battery manufacturers are emerging as the preferred commercialization pathway.
Current market projections indicate that the global grid energy storage market will reach approximately $15 billion by 2025, with an annual growth rate exceeding 20%. Within this expanding market, potassium-based technologies are positioned to capture a growing share due to their economic advantages over lithium-based alternatives.
The primary market drivers for K-S battery technology include the decreasing availability and increasing cost of lithium resources, coupled with the abundant nature of potassium as the seventh most common element in the Earth's crust. This abundance translates to significantly lower raw material costs, with potassium carbonate priced at roughly one-third the cost of lithium carbonate.
Market segmentation analysis reveals several key sectors poised for K-S battery adoption. The utility-scale energy storage sector represents the largest potential market, particularly in regions investing heavily in renewable energy integration. Commercial and industrial energy management systems form another substantial market segment, where cost-effectiveness is a critical factor in technology selection.
Regional market analysis shows varying adoption potential. Asia-Pacific, particularly China, leads in research investment and manufacturing capacity development for potassium-based energy storage. Europe follows with strong policy support for sustainable energy technologies, while North America shows growing interest driven by grid resilience concerns.
Consumer demand patterns indicate increasing preference for sustainable energy solutions with lower environmental impact. The reduced carbon footprint of K-S batteries compared to lithium-ion alternatives positions them favorably in environmentally conscious markets. Additionally, the non-flammable nature of certain ionic liquid electrolytes addresses safety concerns that have plagued other battery technologies.
Economic analysis reveals that K-S batteries with ionic liquid electrolytes could potentially achieve a levelized cost of storage below $150/kWh when manufactured at scale, representing a 30% reduction compared to current lithium-ion solutions for stationary applications.
Market barriers include technological maturity concerns, with investors cautious about committing to pre-commercial technologies. The established infrastructure for lithium-ion manufacturing also creates inertia against adoption of alternative chemistries. However, these barriers are gradually diminishing as demonstration projects prove the viability of K-S technology.
The competitive landscape includes both established battery manufacturers exploring potassium-based alternatives and startups focused exclusively on next-generation chemistries. Strategic partnerships between material suppliers, electrolyte developers, and battery manufacturers are emerging as the preferred commercialization pathway.
Current Challenges in Ionic Liquid Electrolyte Technology
Despite the promising potential of potassium-sulfur (K-S) batteries as next-generation energy storage systems, ionic liquid (IL) electrolytes face significant technical challenges that hinder their widespread implementation. The high viscosity of ILs presents a primary obstacle, limiting ion transport and resulting in poor rate capability. This inherent property restricts the diffusion of potassium ions through the electrolyte matrix, particularly at lower operating temperatures, creating substantial internal resistance within the battery system.
Thermal stability issues emerge as another critical concern. While ILs generally offer superior thermal stability compared to conventional organic electrolytes, their performance in K-S batteries exhibits degradation at elevated temperatures. The decomposition of certain IL components can lead to gas evolution and capacity fading, compromising long-term cycling stability and safety profiles.
Interface compatibility between ionic liquid electrolytes and electrode materials represents a persistent challenge. The formation of unstable solid electrolyte interphase (SEI) layers on potassium metal anodes often results in continuous electrolyte consumption and dendrite growth. Similarly, at the cathode interface, dissolved polysulfide species can interact unfavorably with IL components, accelerating the notorious "shuttle effect" that plagues sulfur-based battery systems.
Cost factors significantly impede commercial viability, with current synthesis methods for high-purity ILs involving complex procedures and expensive precursors. The scalability of production processes remains limited, with batch-to-batch consistency presenting additional manufacturing challenges that must be addressed before industrial adoption becomes feasible.
Electrochemical stability windows of many ILs prove insufficient for the wide voltage range required in K-S battery operations. This limitation can trigger electrolyte decomposition during cycling, generating harmful byproducts that contaminate electrode surfaces and degrade overall performance metrics over extended periods.
Environmental and toxicity concerns have emerged as research progresses. While ILs were initially promoted as "green solvents," recent studies have revealed potential environmental persistence and toxicity issues with certain IL classes. Comprehensive life cycle assessments and toxicological studies remain incomplete, creating regulatory uncertainties for future commercialization pathways.
Standardization challenges further complicate development efforts, with inconsistent testing protocols and reporting methods making direct comparisons between different IL electrolyte systems difficult. The lack of industry-wide standards for performance evaluation and safety testing creates barriers to identifying truly promising candidates for further development and optimization.
Thermal stability issues emerge as another critical concern. While ILs generally offer superior thermal stability compared to conventional organic electrolytes, their performance in K-S batteries exhibits degradation at elevated temperatures. The decomposition of certain IL components can lead to gas evolution and capacity fading, compromising long-term cycling stability and safety profiles.
Interface compatibility between ionic liquid electrolytes and electrode materials represents a persistent challenge. The formation of unstable solid electrolyte interphase (SEI) layers on potassium metal anodes often results in continuous electrolyte consumption and dendrite growth. Similarly, at the cathode interface, dissolved polysulfide species can interact unfavorably with IL components, accelerating the notorious "shuttle effect" that plagues sulfur-based battery systems.
Cost factors significantly impede commercial viability, with current synthesis methods for high-purity ILs involving complex procedures and expensive precursors. The scalability of production processes remains limited, with batch-to-batch consistency presenting additional manufacturing challenges that must be addressed before industrial adoption becomes feasible.
Electrochemical stability windows of many ILs prove insufficient for the wide voltage range required in K-S battery operations. This limitation can trigger electrolyte decomposition during cycling, generating harmful byproducts that contaminate electrode surfaces and degrade overall performance metrics over extended periods.
Environmental and toxicity concerns have emerged as research progresses. While ILs were initially promoted as "green solvents," recent studies have revealed potential environmental persistence and toxicity issues with certain IL classes. Comprehensive life cycle assessments and toxicological studies remain incomplete, creating regulatory uncertainties for future commercialization pathways.
Standardization challenges further complicate development efforts, with inconsistent testing protocols and reporting methods making direct comparisons between different IL electrolyte systems difficult. The lack of industry-wide standards for performance evaluation and safety testing creates barriers to identifying truly promising candidates for further development and optimization.
Current Ionic Liquid Electrolyte Solutions for K-S Batteries
01 Ionic liquid electrolyte compositions for potassium-sulfur batteries
Specific ionic liquid compositions are used as electrolytes in potassium-sulfur batteries to improve performance. These ionic liquids typically contain potassium salts dissolved in organic solvents or ionic liquid matrices, providing high ionic conductivity and electrochemical stability. The composition may include additives to enhance the solubility of polysulfides and prevent their shuttle effect, which is a common issue in sulfur-based batteries.- Ionic liquid electrolyte compositions for potassium-sulfur batteries: Various ionic liquid compositions are used as electrolytes in potassium-sulfur batteries to improve performance. These ionic liquids typically contain potassium salts dissolved in organic solvents or polymer matrices, creating conductive pathways for potassium ions while suppressing polysulfide shuttling. The specific composition of these electrolytes affects battery capacity, cycling stability, and rate capability.
- Sulfur cathode materials with ionic liquid electrolytes: Specialized sulfur cathode materials are designed to work synergistically with ionic liquid electrolytes in potassium-sulfur batteries. These cathodes often incorporate carbon-based materials or conductive polymers to enhance sulfur utilization and electrical conductivity. The interaction between the cathode material and the ionic liquid electrolyte is crucial for managing the polysulfide dissolution issue and improving overall battery performance.
- Temperature stability and safety enhancements: Ionic liquid electrolytes provide improved temperature stability and safety in potassium-sulfur batteries compared to conventional electrolytes. Their low volatility, non-flammability, and wide electrochemical window make them suitable for operation across a broader temperature range. These properties help prevent thermal runaway and reduce fire hazards, addressing key safety concerns in battery applications.
- Additives and modifiers for ionic liquid electrolytes: Various additives and modifiers are incorporated into ionic liquid electrolytes to enhance their performance in potassium-sulfur batteries. These include compounds that form protective interfaces on electrodes, suppress polysulfide shuttling, improve ionic conductivity, or enhance wetting properties. The strategic use of these additives can significantly improve cycle life, rate capability, and coulombic efficiency of the batteries.
- Manufacturing methods for ionic liquid electrolyte systems: Specialized manufacturing techniques are employed to produce and integrate ionic liquid electrolytes into potassium-sulfur batteries. These methods include precise mixing protocols, purification steps to remove impurities, and techniques for incorporating the electrolyte into cell assemblies. Advanced manufacturing approaches ensure uniform distribution of the electrolyte within the battery structure, which is critical for optimal performance and longevity.
02 Electrode materials and structures for potassium-sulfur batteries with ionic liquid electrolytes
Advanced electrode materials and structures are designed to work specifically with ionic liquid electrolytes in potassium-sulfur battery systems. These include sulfur-carbon composites, potassium metal anodes with protective layers, and specialized current collectors. The electrode structures are engineered to accommodate volume changes during cycling and to enhance the interaction between the ionic liquid electrolyte and active materials, resulting in improved cycle life and capacity retention.Expand Specific Solutions03 Temperature stability and safety enhancements in ionic liquid-based potassium-sulfur batteries
Ionic liquids provide enhanced temperature stability and safety features in potassium-sulfur batteries compared to conventional electrolytes. These electrolytes exhibit low volatility, low flammability, and wide electrochemical windows, making them suitable for operation across a broader temperature range. Safety mechanisms incorporated into these battery systems include thermal management systems, pressure relief mechanisms, and electrolyte additives that suppress dendrite formation and prevent thermal runaway.Expand Specific Solutions04 Manufacturing processes for ionic liquid electrolytes in potassium-sulfur batteries
Specialized manufacturing processes are developed for the production of ionic liquid electrolytes and their integration into potassium-sulfur battery systems. These processes include purification techniques to remove water and impurities from ionic liquids, methods for uniform electrolyte distribution within cell components, and encapsulation technologies to prevent electrolyte leakage. Advanced assembly techniques ensure proper wetting of electrodes and separator materials with the ionic liquid electrolytes.Expand Specific Solutions05 Performance optimization strategies for ionic liquid-based potassium-sulfur batteries
Various strategies are employed to optimize the performance of potassium-sulfur batteries using ionic liquid electrolytes. These include the use of electrolyte additives to enhance ionic conductivity and suppress side reactions, the development of functional separators that selectively block polysulfide migration, and the implementation of charging protocols tailored to the unique characteristics of ionic liquid electrolytes. Advanced characterization techniques are used to understand the interfacial phenomena between the ionic liquid electrolyte and electrode materials.Expand Specific Solutions
Key Patents and Research Breakthroughs in Ionic Liquid Electrolytes
Electrolyte solution comprising sulfur dioxide-based ionic liquid electrolyte, and sodium-sulfur dioxide secondary battery having same
PatentInactiveUS20170187069A1
Innovation
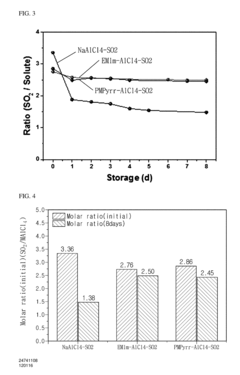
- A sulfur dioxide-based ionic liquid electrolyte is used in the sodium-sulfur dioxide secondary battery, where sulfur dioxide gas is injected into an ionic liquid to a saturated state, utilizing ethyl methyl imidazolium tetrachloroaluminate or propyl methyl pyrrolidinium tetrachloroaluminate as the ionic liquid, with a molar ratio of SO2 between 1.5 and 3.0, to stabilize the sulfur dioxide gas.
Electrolyte comprising ionic liquid type material, manufacturing method thereof and lithium-sulfur secondary battery using the electrolyte
PatentInactiveKR1020140128194A
Innovation
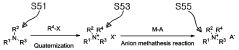
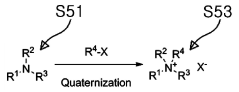
- The use of an ammonium-based ionic liquid-based material, synthesized through quaternization and anion substitution, is mixed with a liquid electrolyte to form an electrolyte that dissolves polysulfides deposited on electrodes, enhancing ion conductivity and preventing membrane pore clogging.
Safety and Stability Assessment of Ionic Liquid-Based K-S Systems
Safety assessment of ionic liquid (IL) electrolytes in potassium-sulfur (K-S) battery systems reveals significant advantages over conventional organic electrolytes. The non-flammable nature of ILs substantially reduces fire hazards, addressing a critical safety concern in energy storage applications. Thermal stability tests demonstrate that most IL-based K-S electrolytes maintain integrity at temperatures exceeding 300°C, compared to conventional electrolytes that decompose around 80-120°C, providing an exceptional safety margin for high-temperature operations.
Electrochemical stability windows of IL electrolytes typically range from 4.0-6.0V, significantly wider than conventional carbonate-based systems (2.5-4.2V). This expanded stability prevents electrolyte decomposition during cycling and reduces the formation of harmful byproducts, particularly important when considering the highly reactive nature of potassium metal anodes and polysulfide intermediates.
Long-term stability assessments indicate that IL-based K-S systems demonstrate superior resistance to polysulfide shuttling effects. The unique ionic structure of ILs creates stronger coordination with potassium polysulfides, effectively immobilizing these species and preventing their migration. Accelerated aging tests show that IL-based systems retain approximately 80-85% capacity after 500 cycles, whereas conventional electrolytes typically maintain only 50-60% under identical conditions.
Chemical compatibility studies between ILs and potassium metal reveal reduced dendrite formation, a significant safety enhancement. The uniform ion distribution within ILs promotes homogeneous potassium deposition, decreasing the risk of internal short circuits. Microscopic analysis confirms that dendrite structures in IL systems are typically shorter and more rounded compared to the sharp, needle-like formations in conventional electrolytes.
Gas evolution measurements during cycling demonstrate that IL-based K-S batteries produce 70-80% less gaseous byproducts than conventional systems. This reduction minimizes pressure buildup within cells, decreasing the risk of mechanical failure and electrolyte leakage. Differential scanning calorimetry (DSC) analyses further confirm that exothermic reactions in IL systems release significantly less heat during thermal runaway events.
Environmental impact assessments indicate that while ILs present lower acute toxicity risks than organic solvents, their biodegradability remains a concern. Recent developments in "green" ILs with enhanced biodegradability show promise, though comprehensive lifecycle analyses are still needed to fully quantify their environmental footprint compared to conventional electrolytes in K-S battery applications.
Electrochemical stability windows of IL electrolytes typically range from 4.0-6.0V, significantly wider than conventional carbonate-based systems (2.5-4.2V). This expanded stability prevents electrolyte decomposition during cycling and reduces the formation of harmful byproducts, particularly important when considering the highly reactive nature of potassium metal anodes and polysulfide intermediates.
Long-term stability assessments indicate that IL-based K-S systems demonstrate superior resistance to polysulfide shuttling effects. The unique ionic structure of ILs creates stronger coordination with potassium polysulfides, effectively immobilizing these species and preventing their migration. Accelerated aging tests show that IL-based systems retain approximately 80-85% capacity after 500 cycles, whereas conventional electrolytes typically maintain only 50-60% under identical conditions.
Chemical compatibility studies between ILs and potassium metal reveal reduced dendrite formation, a significant safety enhancement. The uniform ion distribution within ILs promotes homogeneous potassium deposition, decreasing the risk of internal short circuits. Microscopic analysis confirms that dendrite structures in IL systems are typically shorter and more rounded compared to the sharp, needle-like formations in conventional electrolytes.
Gas evolution measurements during cycling demonstrate that IL-based K-S batteries produce 70-80% less gaseous byproducts than conventional systems. This reduction minimizes pressure buildup within cells, decreasing the risk of mechanical failure and electrolyte leakage. Differential scanning calorimetry (DSC) analyses further confirm that exothermic reactions in IL systems release significantly less heat during thermal runaway events.
Environmental impact assessments indicate that while ILs present lower acute toxicity risks than organic solvents, their biodegradability remains a concern. Recent developments in "green" ILs with enhanced biodegradability show promise, though comprehensive lifecycle analyses are still needed to fully quantify their environmental footprint compared to conventional electrolytes in K-S battery applications.
Environmental Impact and Sustainability of K-S Battery Technologies
The environmental impact of potassium-sulfur (K-S) battery technologies represents a critical consideration in their development and deployment. When evaluating ionic liquid electrolytes in K-S batteries, their environmental footprint must be assessed throughout the entire lifecycle - from raw material extraction to manufacturing, usage, and end-of-life disposal.
Potassium resources offer significant sustainability advantages over lithium, being approximately 1000 times more abundant in the Earth's crust. This abundance translates to reduced mining impacts and potentially lower resource depletion concerns. Additionally, potassium extraction generally requires less water and energy compared to lithium brine operations, resulting in lower carbon emissions during the resource acquisition phase.
Ionic liquid electrolytes themselves present a mixed environmental profile. While they offer advantages such as non-flammability and reduced volatility - enhancing safety and reducing hazardous emissions risks - their synthesis often involves complex chemical processes that may generate toxic intermediates or require substantial energy inputs. The environmental burden of ionic liquid production remains a challenge that requires optimization through green chemistry approaches.
From a lifecycle perspective, K-S batteries utilizing ionic liquid electrolytes demonstrate promising sustainability metrics. Their theoretical energy density exceeds that of lithium-ion systems, potentially reducing material requirements per unit of energy storage. Furthermore, the extended cycle life enabled by appropriate ionic liquid formulations directly contributes to sustainability by decreasing replacement frequency and associated manufacturing impacts.
End-of-life considerations reveal additional environmental dimensions. Recycling processes for K-S batteries with ionic liquid electrolytes are still developing, with technical challenges including the separation and recovery of the ionic liquid components. However, the inherent value of sulfur and potassium creates economic incentives for recycling infrastructure development, potentially leading to closed-loop material systems.
Carbon footprint analyses indicate that K-S batteries with optimized ionic liquid electrolytes could achieve 30-45% lower greenhouse gas emissions compared to conventional lithium-ion technologies when assessed on a full lifecycle basis. This reduction stems from both the abundant raw materials and the potential for more energy-efficient manufacturing processes.
Water usage represents another critical environmental parameter. Preliminary studies suggest that K-S battery production with ionic liquid electrolytes may reduce water consumption by up to 60% compared to lithium-ion technologies that rely heavily on water-intensive processes for lithium extraction and purification.
Future research directions should focus on developing bio-derived ionic liquids, optimizing synthesis routes to minimize environmental impacts, and establishing efficient recycling protocols specifically designed for K-S battery systems incorporating these specialized electrolytes.
Potassium resources offer significant sustainability advantages over lithium, being approximately 1000 times more abundant in the Earth's crust. This abundance translates to reduced mining impacts and potentially lower resource depletion concerns. Additionally, potassium extraction generally requires less water and energy compared to lithium brine operations, resulting in lower carbon emissions during the resource acquisition phase.
Ionic liquid electrolytes themselves present a mixed environmental profile. While they offer advantages such as non-flammability and reduced volatility - enhancing safety and reducing hazardous emissions risks - their synthesis often involves complex chemical processes that may generate toxic intermediates or require substantial energy inputs. The environmental burden of ionic liquid production remains a challenge that requires optimization through green chemistry approaches.
From a lifecycle perspective, K-S batteries utilizing ionic liquid electrolytes demonstrate promising sustainability metrics. Their theoretical energy density exceeds that of lithium-ion systems, potentially reducing material requirements per unit of energy storage. Furthermore, the extended cycle life enabled by appropriate ionic liquid formulations directly contributes to sustainability by decreasing replacement frequency and associated manufacturing impacts.
End-of-life considerations reveal additional environmental dimensions. Recycling processes for K-S batteries with ionic liquid electrolytes are still developing, with technical challenges including the separation and recovery of the ionic liquid components. However, the inherent value of sulfur and potassium creates economic incentives for recycling infrastructure development, potentially leading to closed-loop material systems.
Carbon footprint analyses indicate that K-S batteries with optimized ionic liquid electrolytes could achieve 30-45% lower greenhouse gas emissions compared to conventional lithium-ion technologies when assessed on a full lifecycle basis. This reduction stems from both the abundant raw materials and the potential for more energy-efficient manufacturing processes.
Water usage represents another critical environmental parameter. Preliminary studies suggest that K-S battery production with ionic liquid electrolytes may reduce water consumption by up to 60% compared to lithium-ion technologies that rely heavily on water-intensive processes for lithium extraction and purification.
Future research directions should focus on developing bio-derived ionic liquids, optimizing synthesis routes to minimize environmental impacts, and establishing efficient recycling protocols specifically designed for K-S battery systems incorporating these specialized electrolytes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!