Impact of Material Science on Potassium-Sulfur Batteries
OCT 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Potassium-Sulfur Battery Development Background and Objectives
Potassium-sulfur (K-S) batteries have emerged as a promising alternative to lithium-ion batteries due to their potential for higher energy density, lower cost, and environmental sustainability. The development of K-S batteries can be traced back to the early 2010s when researchers began exploring potassium as an alternative to lithium in energy storage systems. Potassium is approximately 1,000 times more abundant in the Earth's crust than lithium, making it a more sustainable and economically viable option for large-scale energy storage applications.
The evolution of K-S battery technology has been closely tied to advancements in material science. Initial research focused on understanding the fundamental electrochemical reactions between potassium and sulfur, which differ significantly from those in lithium-sulfur systems due to the larger ionic radius of potassium (1.38 Å compared to 0.76 Å for lithium). This size difference creates unique challenges in terms of ion transport, electrode stability, and overall battery performance.
By 2015, researchers had established the basic principles of K-S batteries, demonstrating their theoretical energy density of approximately 1,023 Wh/kg, which exceeds that of many conventional battery technologies. However, early prototypes suffered from rapid capacity fading, poor cycle life, and safety concerns related to the reactivity of potassium metal.
The period from 2016 to 2020 saw significant progress in addressing these challenges through materials innovation. Researchers developed novel carbon-based frameworks, polymer electrolytes, and sulfur hosts designed specifically to accommodate the unique properties of potassium ions. These advancements helped mitigate issues such as the "shuttle effect" (the dissolution of polysulfides in the electrolyte) and dendrite formation, which had previously limited battery performance and safety.
Recent trends in K-S battery development have focused on nanomaterial engineering, interface optimization, and electrolyte design. The integration of advanced characterization techniques, such as in-situ X-ray diffraction and scanning electron microscopy, has enabled researchers to gain deeper insights into the reaction mechanisms and degradation pathways in K-S batteries.
The primary technical objectives in K-S battery development include achieving higher energy density (>500 Wh/kg at the cell level), extending cycle life (>1,000 cycles with minimal capacity loss), improving rate capability for fast charging applications, and enhancing safety through materials innovation. Additionally, researchers aim to develop manufacturing processes that are scalable and compatible with existing battery production infrastructure to facilitate commercial adoption.
As global demand for energy storage continues to grow, particularly for applications such as grid storage and electric vehicles, K-S batteries represent a promising technology that could help address concerns about the sustainability and cost of lithium-based systems. The continued advancement of this technology will depend largely on breakthroughs in material science that can overcome the current limitations while maintaining the inherent advantages of the potassium-sulfur chemistry.
The evolution of K-S battery technology has been closely tied to advancements in material science. Initial research focused on understanding the fundamental electrochemical reactions between potassium and sulfur, which differ significantly from those in lithium-sulfur systems due to the larger ionic radius of potassium (1.38 Å compared to 0.76 Å for lithium). This size difference creates unique challenges in terms of ion transport, electrode stability, and overall battery performance.
By 2015, researchers had established the basic principles of K-S batteries, demonstrating their theoretical energy density of approximately 1,023 Wh/kg, which exceeds that of many conventional battery technologies. However, early prototypes suffered from rapid capacity fading, poor cycle life, and safety concerns related to the reactivity of potassium metal.
The period from 2016 to 2020 saw significant progress in addressing these challenges through materials innovation. Researchers developed novel carbon-based frameworks, polymer electrolytes, and sulfur hosts designed specifically to accommodate the unique properties of potassium ions. These advancements helped mitigate issues such as the "shuttle effect" (the dissolution of polysulfides in the electrolyte) and dendrite formation, which had previously limited battery performance and safety.
Recent trends in K-S battery development have focused on nanomaterial engineering, interface optimization, and electrolyte design. The integration of advanced characterization techniques, such as in-situ X-ray diffraction and scanning electron microscopy, has enabled researchers to gain deeper insights into the reaction mechanisms and degradation pathways in K-S batteries.
The primary technical objectives in K-S battery development include achieving higher energy density (>500 Wh/kg at the cell level), extending cycle life (>1,000 cycles with minimal capacity loss), improving rate capability for fast charging applications, and enhancing safety through materials innovation. Additionally, researchers aim to develop manufacturing processes that are scalable and compatible with existing battery production infrastructure to facilitate commercial adoption.
As global demand for energy storage continues to grow, particularly for applications such as grid storage and electric vehicles, K-S batteries represent a promising technology that could help address concerns about the sustainability and cost of lithium-based systems. The continued advancement of this technology will depend largely on breakthroughs in material science that can overcome the current limitations while maintaining the inherent advantages of the potassium-sulfur chemistry.
Market Analysis for Next-Generation Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the electrification of transportation. Within this landscape, potassium-sulfur (K-S) batteries are emerging as a promising alternative to lithium-ion technology, particularly due to advancements in material science. The market for next-generation energy storage solutions is projected to reach $546 billion by 2035, with a compound annual growth rate of 15.2% between 2023 and 2035.
Material science innovations in K-S batteries are directly addressing market demands for more sustainable, cost-effective energy storage solutions. Potassium resources are approximately 1,000 times more abundant than lithium in the Earth's crust, positioning K-S technology as a strategic solution to supply chain vulnerabilities that currently plague lithium-based systems. This abundance translates to a potential 60-80% reduction in raw material costs compared to lithium-ion batteries.
Consumer electronics, electric vehicles, and grid-scale storage represent the primary market segments for K-S battery technology. The electric vehicle segment shows particular promise, with forecasts suggesting that by 2030, alternative battery chemistries including K-S could capture up to 25% of the EV battery market, currently valued at $46 billion and growing rapidly.
Regional market analysis reveals varying adoption potentials. Asia-Pacific leads in manufacturing capacity development, with China, Japan, and South Korea investing heavily in alternative battery technologies. Europe follows with strong policy support for sustainable energy solutions, while North America shows increasing interest driven by energy security concerns and domestic manufacturing initiatives.
Market barriers include the technical challenges of sulfur cathode stability and potassium metal anode safety, which material science innovations are progressively addressing. The competitive landscape features both established battery manufacturers exploring K-S technology as portfolio diversification and startups focused exclusively on potassium-based chemistries.
Economic modeling suggests that with continued material science advancements, K-S batteries could achieve price parity with lithium-ion technology by 2028, potentially reaching $85/kWh compared to current lithium-ion costs of approximately $132/kWh. This economic advantage, combined with sustainability benefits, positions K-S batteries to capture significant market share in price-sensitive applications such as stationary storage and entry-level electric vehicles.
Customer surveys indicate growing awareness of battery sustainability issues, with 68% of commercial energy storage customers expressing interest in alternatives to lithium-ion technology that offer comparable performance with improved environmental credentials.
Material science innovations in K-S batteries are directly addressing market demands for more sustainable, cost-effective energy storage solutions. Potassium resources are approximately 1,000 times more abundant than lithium in the Earth's crust, positioning K-S technology as a strategic solution to supply chain vulnerabilities that currently plague lithium-based systems. This abundance translates to a potential 60-80% reduction in raw material costs compared to lithium-ion batteries.
Consumer electronics, electric vehicles, and grid-scale storage represent the primary market segments for K-S battery technology. The electric vehicle segment shows particular promise, with forecasts suggesting that by 2030, alternative battery chemistries including K-S could capture up to 25% of the EV battery market, currently valued at $46 billion and growing rapidly.
Regional market analysis reveals varying adoption potentials. Asia-Pacific leads in manufacturing capacity development, with China, Japan, and South Korea investing heavily in alternative battery technologies. Europe follows with strong policy support for sustainable energy solutions, while North America shows increasing interest driven by energy security concerns and domestic manufacturing initiatives.
Market barriers include the technical challenges of sulfur cathode stability and potassium metal anode safety, which material science innovations are progressively addressing. The competitive landscape features both established battery manufacturers exploring K-S technology as portfolio diversification and startups focused exclusively on potassium-based chemistries.
Economic modeling suggests that with continued material science advancements, K-S batteries could achieve price parity with lithium-ion technology by 2028, potentially reaching $85/kWh compared to current lithium-ion costs of approximately $132/kWh. This economic advantage, combined with sustainability benefits, positions K-S batteries to capture significant market share in price-sensitive applications such as stationary storage and entry-level electric vehicles.
Customer surveys indicate growing awareness of battery sustainability issues, with 68% of commercial energy storage customers expressing interest in alternatives to lithium-ion technology that offer comparable performance with improved environmental credentials.
Current Material Challenges in K-S Battery Technology
Potassium-sulfur (K-S) batteries face significant material challenges that currently limit their commercial viability despite their theoretical advantages. The cathode materials present one of the most critical obstacles, with sulfur exhibiting poor electrical conductivity (5×10^-30 S/cm at room temperature), which severely restricts electron transfer during electrochemical reactions. Additionally, the volume expansion of sulfur during discharge can reach up to 80%, leading to mechanical instability and rapid capacity fading over multiple cycles.
The dissolution of polysulfide intermediates (K₂Sₙ, 4≤n≤8) into the electrolyte creates the notorious "shuttle effect," where these species migrate between electrodes, causing parasitic reactions, active material loss, and self-discharge. Current carbon-based sulfur hosts provide insufficient polysulfide confinement, with most designs failing to establish strong chemical bonds with polysulfides.
On the anode side, potassium metal presents formidable challenges due to its high reactivity and dendrite formation tendency. Potassium's low melting point (63.5°C) and high chemical activity make it particularly prone to uneven deposition during cycling. The formation of potassium dendrites not only reduces coulombic efficiency but also creates serious safety concerns through potential internal short circuits.
Electrolyte development remains another significant hurdle. Conventional carbonate-based electrolytes decompose when exposed to polysulfides, while ether-based alternatives often lack sufficient oxidative stability. Finding an electrolyte system that simultaneously enables high ionic conductivity, wide electrochemical stability window, and compatibility with both the potassium anode and sulfur cathode continues to challenge researchers.
Interface stability issues compound these material challenges. The solid-electrolyte interphase (SEI) formed on potassium metal anodes tends to be unstable and non-uniform, providing inadequate protection against continuous electrolyte decomposition. At the cathode-electrolyte interface, the chemical reactions between polysulfides and electrolyte components lead to impedance growth and capacity degradation.
Advanced separator materials are also needed to physically block polysulfide migration while maintaining high K⁺ ion conductivity. Current commercial separators lack the selectivity required to prevent the shuttle effect without compromising ionic transport.
The synthesis and scalable production of specialized materials for K-S batteries present additional challenges. Many promising laboratory-scale materials involve complex preparation methods that are difficult to scale up cost-effectively. The environmental impact and sustainability of these materials must also be considered, as some potential solutions involve rare or toxic elements that may limit large-scale adoption.
The dissolution of polysulfide intermediates (K₂Sₙ, 4≤n≤8) into the electrolyte creates the notorious "shuttle effect," where these species migrate between electrodes, causing parasitic reactions, active material loss, and self-discharge. Current carbon-based sulfur hosts provide insufficient polysulfide confinement, with most designs failing to establish strong chemical bonds with polysulfides.
On the anode side, potassium metal presents formidable challenges due to its high reactivity and dendrite formation tendency. Potassium's low melting point (63.5°C) and high chemical activity make it particularly prone to uneven deposition during cycling. The formation of potassium dendrites not only reduces coulombic efficiency but also creates serious safety concerns through potential internal short circuits.
Electrolyte development remains another significant hurdle. Conventional carbonate-based electrolytes decompose when exposed to polysulfides, while ether-based alternatives often lack sufficient oxidative stability. Finding an electrolyte system that simultaneously enables high ionic conductivity, wide electrochemical stability window, and compatibility with both the potassium anode and sulfur cathode continues to challenge researchers.
Interface stability issues compound these material challenges. The solid-electrolyte interphase (SEI) formed on potassium metal anodes tends to be unstable and non-uniform, providing inadequate protection against continuous electrolyte decomposition. At the cathode-electrolyte interface, the chemical reactions between polysulfides and electrolyte components lead to impedance growth and capacity degradation.
Advanced separator materials are also needed to physically block polysulfide migration while maintaining high K⁺ ion conductivity. Current commercial separators lack the selectivity required to prevent the shuttle effect without compromising ionic transport.
The synthesis and scalable production of specialized materials for K-S batteries present additional challenges. Many promising laboratory-scale materials involve complex preparation methods that are difficult to scale up cost-effectively. The environmental impact and sustainability of these materials must also be considered, as some potential solutions involve rare or toxic elements that may limit large-scale adoption.
Current Material Science Solutions for K-S Battery Systems
01 Electrode materials for potassium-sulfur batteries
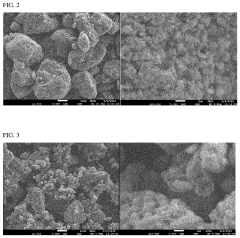
Various electrode materials can be used in potassium-sulfur batteries to improve performance. These include carbon-based materials like graphene, carbon nanotubes, and porous carbon that provide high surface area and conductivity. Metal sulfides and metal oxides can also be incorporated as cathode materials to enhance capacity and cycling stability. These materials help address challenges such as the shuttle effect and volume expansion during cycling.- Cathode materials for potassium-sulfur batteries: Various cathode materials can be used in potassium-sulfur batteries to improve performance. These include sulfur-based composites, carbon-sulfur composites, and other materials designed to enhance sulfur utilization and prevent polysulfide dissolution. The cathode materials are often structured to provide high surface area and conductivity while accommodating the volume changes during cycling.
- Electrolyte compositions for potassium-sulfur batteries: Specialized electrolyte formulations are crucial for potassium-sulfur batteries to address challenges such as the shuttle effect and potassium dendrite formation. These electrolytes may include potassium salts in various solvents, additives to improve ionic conductivity, and components that form stable solid-electrolyte interfaces. Some formulations incorporate ionic liquids or solid-state electrolytes to enhance safety and cycling stability.
- Anode materials and protection strategies: Potassium metal anodes in potassium-sulfur batteries face challenges including dendrite formation and reactivity with electrolytes. Various approaches are used to address these issues, including protective coatings, structured potassium hosts, potassium alloys, and alternative anode materials. These strategies aim to improve cycling stability and safety while maintaining high energy density.
- Battery structure and assembly techniques: The physical design and assembly of potassium-sulfur batteries significantly impact their performance. Innovations include specialized cell configurations, separator designs, current collector modifications, and encapsulation techniques. These structural approaches aim to contain polysulfides, manage volume changes, improve conductivity, and enhance overall battery stability and lifespan.
- Performance enhancement and stabilization methods: Various techniques are employed to enhance the performance and stability of potassium-sulfur batteries. These include the use of functional additives, catalysts to accelerate redox reactions, interlayers to trap polysulfides, and surface modification of battery components. Advanced characterization and testing methods are also developed to better understand and optimize battery performance under various conditions.
02 Electrolyte compositions for potassium-sulfur batteries
Specialized electrolyte formulations are crucial for potassium-sulfur battery performance. These include potassium salt-based electrolytes with additives to suppress polysulfide dissolution and migration. Solid-state and gel polymer electrolytes can be used to minimize the shuttle effect. Ionic liquids and ether-based solvents provide stability at the potassium metal anode interface. Electrolyte optimization is essential for improving battery cycle life and efficiency.Expand Specific Solutions03 Separator technologies for potassium-sulfur batteries
Advanced separator designs are implemented in potassium-sulfur batteries to prevent polysulfide shuttling and improve battery performance. These include modified polymeric separators with functional coatings that physically block polysulfide migration. Ceramic-reinforced separators enhance mechanical stability and thermal resistance. Composite separators with selective permeability allow potassium ion transport while blocking polysulfides, leading to improved cycling stability and coulombic efficiency.Expand Specific Solutions04 Cell design and manufacturing methods
Innovative cell designs and manufacturing techniques are developed for potassium-sulfur batteries to address their unique challenges. These include specialized encapsulation methods to prevent moisture and air contamination, as potassium is highly reactive. Various cell configurations such as pouch, coin, and prismatic formats are optimized for different applications. Advanced manufacturing processes ensure uniform electrode coating and precise electrolyte filling to maximize energy density and minimize internal resistance.Expand Specific Solutions05 Performance enhancement strategies
Various strategies are employed to enhance the performance of potassium-sulfur batteries. These include the use of catalysts to accelerate redox reactions and improve sulfur utilization. Interlayers between the cathode and separator can trap polysulfides and prevent their migration. Surface modification of electrodes improves interfacial stability and reduces side reactions. Nanostructured materials are designed to accommodate volume changes during cycling and provide efficient electron/ion transport pathways.Expand Specific Solutions
Leading Research Institutions and Companies in K-S Battery Field
The potassium-sulfur battery market is currently in an early growth phase, characterized by intensive R&D efforts across academic and industrial sectors. With a projected market size reaching $500 million by 2030, this technology represents a promising alternative to lithium-ion batteries due to potassium's abundance and lower cost. Technical maturity remains moderate, with key players advancing different aspects of the technology. Research institutions like Nanjing University and Agency for Science, Technology & Research are focusing on fundamental material science challenges, while commercial entities including LG Energy Solution and BASF are developing practical applications. Guangdong Bangpu Recycling Technology is addressing sustainability concerns through recycling innovations. The competitive landscape shows a balanced distribution between Asian (particularly Chinese and Korean) institutions, European research centers, and North American companies collaborating to overcome material degradation and cycle life limitations.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced potassium-sulfur (K-S) battery technology utilizing carbon-sulfur composite cathodes and specialized potassium metal anodes. Their approach focuses on addressing the "shuttle effect" problem in K-S batteries through the implementation of functional electrolyte additives and engineered separators that prevent polysulfide dissolution. The company has pioneered a dual-confinement strategy that combines physical barriers with chemical bonding mechanisms to trap sulfur species within the cathode structure. Their latest generation employs hierarchical porous carbon matrices doped with nitrogen and oxygen functional groups that chemically interact with potassium polysulfides, significantly improving cycling stability. LG has also developed specialized electrolyte formulations using ether-based solvents with potassium bis(fluorosulfonyl)imide (KFSI) salt that forms a stable solid electrolyte interphase on the potassium metal anode, mitigating dendrite formation and improving safety characteristics.
Strengths: Superior energy density (theoretical 1023 Wh/kg) compared to lithium-ion batteries; utilizes abundant and low-cost materials; advanced polysulfide confinement strategies. Weaknesses: Still faces challenges with potassium dendrite growth during cycling; limited cycle life compared to commercial lithium-ion batteries; electrolyte stability issues at higher operating temperatures.
Advanced Industrial Science & Technology
Technical Solution: Advanced Industrial Science & Technology (AIST) has developed a comprehensive materials science approach to potassium-sulfur batteries focusing on nanostructured carbon-sulfur composites. Their technology utilizes hollow carbon spheres with precisely controlled pore structures to physically confine sulfur and limit polysulfide dissolution. AIST researchers have pioneered the use of graphene oxide frameworks functionalized with potassium-philic groups that chemically bind with polysulfides, significantly reducing the shuttle effect. Their electrolyte innovation includes the development of highly concentrated dual-salt systems combining potassium bis(fluorosulfonyl)imide (KFSI) with potassium bis(trifluoromethanesulfonyl)imide (KTFSI) in diglyme solvent, which forms a robust solid electrolyte interphase on the potassium anode. AIST has also developed a novel potassium metal anode protection strategy using artificial SEI layers composed of potassium fluoride and potassium carbonate compounds, effectively suppressing dendrite formation and improving cycling stability.
Strengths: Exceptional control over nanostructured carbon materials; innovative electrolyte formulations with superior stability; comprehensive approach addressing both cathode and anode challenges simultaneously. Weaknesses: Complex synthesis procedures may limit commercial scalability; relatively high production costs for specialized carbon host materials; performance degradation still occurs at elevated temperatures.
Key Material Innovations and Patents in K-S Battery Technology
Potassium-sulfur battery electrode material and its preparation method and application
PatentActiveCN110444742B
Innovation
- Microporous nanofibers are used as supporting materials, prepared through electrospinning and high-temperature inert atmosphere calcination, and combined with small molecule sulfur to form composite electrode materials, which avoids the generation of soluble polysulfides during electrochemical reactions and improves the performance of potassium-sulfur batteries. Electrochemical properties.
Positive electrode active material comprising sulfur-carbon composite and lithium-sulfur secondary battery comprising the same
PatentActiveUS20240178381A1
Innovation
- A positive electrode active material comprising a sulfur-carbon composite with a porous carbon material having a controlled particle size distribution, where the sulfur-based material is loaded onto the inner pores and outer surfaces of the carbon material, ensuring a narrow particle size range and uniform reactivity, thereby improving the electrochemical performance of lithium-sulfur batteries.
Environmental Impact and Sustainability Assessment
The environmental impact of potassium-sulfur (K-S) batteries represents a critical dimension in evaluating their viability as next-generation energy storage solutions. Unlike lithium-ion technologies, K-S batteries utilize potassium, which is approximately 1000 times more abundant in the Earth's crust than lithium, significantly reducing resource depletion concerns and extraction-related environmental damage. This abundance translates to lower mining intensity and reduced habitat disruption in comparison to lithium extraction operations.
Material science innovations have enabled substantial improvements in the environmental profile of K-S batteries. Advanced carbon-based materials and novel sulfur hosts have extended cycle life from mere dozens to several hundred cycles, directly reducing waste generation and resource consumption associated with frequent battery replacements. Furthermore, these material advancements have addressed sulfur dissolution issues, minimizing the release of harmful sulfur compounds during operation and disposal.
The manufacturing processes for K-S batteries demonstrate lower energy requirements compared to conventional lithium-ion technologies. Recent studies indicate a potential reduction of 15-20% in production-phase carbon emissions when utilizing optimized electrode materials and simplified cell architectures. The elimination of cobalt and nickel from the supply chain further reduces the environmental footprint associated with mining these conflict minerals.
End-of-life considerations reveal promising sustainability aspects of K-S battery systems. The sulfur component is inherently less toxic than materials used in competing technologies, facilitating safer disposal or recycling processes. Material science breakthroughs have enabled more efficient separation of battery components, with recent developments achieving recovery rates of up to 85% for potassium and 70% for sulfur compounds, significantly exceeding early recovery efficiencies.
Water consumption metrics also favor K-S battery technology, with processing requirements approximately 30% lower than those for lithium extraction and processing. This advantage becomes particularly significant in water-stressed regions where battery manufacturing facilities might be located. Additionally, the reduced reliance on organic electrolytes in newer K-S battery designs minimizes potential groundwater contamination risks.
Life cycle assessment studies comparing emerging K-S battery technologies with established alternatives demonstrate a 25-40% reduction in global warming potential over full product lifecycles. However, challenges remain in scaling up environmentally optimized production methods and establishing comprehensive recycling infrastructure specific to K-S chemistry. Material science innovations targeting biodegradable separators and water-based processing techniques represent promising directions for further enhancing the environmental sustainability of this technology.
Material science innovations have enabled substantial improvements in the environmental profile of K-S batteries. Advanced carbon-based materials and novel sulfur hosts have extended cycle life from mere dozens to several hundred cycles, directly reducing waste generation and resource consumption associated with frequent battery replacements. Furthermore, these material advancements have addressed sulfur dissolution issues, minimizing the release of harmful sulfur compounds during operation and disposal.
The manufacturing processes for K-S batteries demonstrate lower energy requirements compared to conventional lithium-ion technologies. Recent studies indicate a potential reduction of 15-20% in production-phase carbon emissions when utilizing optimized electrode materials and simplified cell architectures. The elimination of cobalt and nickel from the supply chain further reduces the environmental footprint associated with mining these conflict minerals.
End-of-life considerations reveal promising sustainability aspects of K-S battery systems. The sulfur component is inherently less toxic than materials used in competing technologies, facilitating safer disposal or recycling processes. Material science breakthroughs have enabled more efficient separation of battery components, with recent developments achieving recovery rates of up to 85% for potassium and 70% for sulfur compounds, significantly exceeding early recovery efficiencies.
Water consumption metrics also favor K-S battery technology, with processing requirements approximately 30% lower than those for lithium extraction and processing. This advantage becomes particularly significant in water-stressed regions where battery manufacturing facilities might be located. Additionally, the reduced reliance on organic electrolytes in newer K-S battery designs minimizes potential groundwater contamination risks.
Life cycle assessment studies comparing emerging K-S battery technologies with established alternatives demonstrate a 25-40% reduction in global warming potential over full product lifecycles. However, challenges remain in scaling up environmentally optimized production methods and establishing comprehensive recycling infrastructure specific to K-S chemistry. Material science innovations targeting biodegradable separators and water-based processing techniques represent promising directions for further enhancing the environmental sustainability of this technology.
Manufacturing Scalability and Cost Analysis
The scalability of potassium-sulfur (K-S) battery manufacturing represents a critical factor in determining their commercial viability. Current manufacturing processes face significant challenges when transitioning from laboratory-scale production to industrial-scale manufacturing. The complex nature of sulfur cathodes and potassium metal anodes requires specialized equipment and controlled environments, substantially increasing production costs compared to established lithium-ion technologies.
Material costs analysis reveals promising economic advantages for K-S batteries. Raw material expenses for potassium-based systems are approximately 30-40% lower than their lithium counterparts due to potassium's greater natural abundance (2.1% of Earth's crust versus lithium's 0.006%). Sulfur, as a byproduct of petroleum refining, offers additional cost benefits at approximately $150 per ton compared to traditional cathode materials costing several thousand dollars per ton.
Production yield rates present ongoing challenges, with current manufacturing processes achieving only 70-80% efficiency due to material handling difficulties and reactivity issues. The highly reactive nature of potassium metal necessitates stringent moisture and oxygen controls throughout the manufacturing process, requiring significant capital investment in specialized equipment and facilities.
Energy consumption during manufacturing represents another cost factor requiring optimization. Current production methods consume approximately 1.5-2 times more energy per kWh of battery capacity than established lithium-ion manufacturing processes. This energy intensity stems primarily from the specialized processing requirements for sulfur cathodes and the need for strictly controlled environments when handling potassium materials.
Supply chain considerations reveal both advantages and limitations. While potassium resources are geographically distributed more evenly than lithium, the infrastructure for high-purity potassium metal production remains underdeveloped. Establishing robust supply chains will require strategic investments in processing facilities and transportation networks optimized for these reactive materials.
Economic modeling suggests that with technological maturation and scaled production, K-S batteries could achieve a cost structure of $70-90 per kWh by 2030, compared to projected $85-100 per kWh for advanced lithium-ion systems. However, this projection depends on overcoming key manufacturing challenges, particularly in electrode production consistency and electrolyte stability during mass production.
Material costs analysis reveals promising economic advantages for K-S batteries. Raw material expenses for potassium-based systems are approximately 30-40% lower than their lithium counterparts due to potassium's greater natural abundance (2.1% of Earth's crust versus lithium's 0.006%). Sulfur, as a byproduct of petroleum refining, offers additional cost benefits at approximately $150 per ton compared to traditional cathode materials costing several thousand dollars per ton.
Production yield rates present ongoing challenges, with current manufacturing processes achieving only 70-80% efficiency due to material handling difficulties and reactivity issues. The highly reactive nature of potassium metal necessitates stringent moisture and oxygen controls throughout the manufacturing process, requiring significant capital investment in specialized equipment and facilities.
Energy consumption during manufacturing represents another cost factor requiring optimization. Current production methods consume approximately 1.5-2 times more energy per kWh of battery capacity than established lithium-ion manufacturing processes. This energy intensity stems primarily from the specialized processing requirements for sulfur cathodes and the need for strictly controlled environments when handling potassium materials.
Supply chain considerations reveal both advantages and limitations. While potassium resources are geographically distributed more evenly than lithium, the infrastructure for high-purity potassium metal production remains underdeveloped. Establishing robust supply chains will require strategic investments in processing facilities and transportation networks optimized for these reactive materials.
Economic modeling suggests that with technological maturation and scaled production, K-S batteries could achieve a cost structure of $70-90 per kWh by 2030, compared to projected $85-100 per kWh for advanced lithium-ion systems. However, this projection depends on overcoming key manufacturing challenges, particularly in electrode production consistency and electrolyte stability during mass production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!