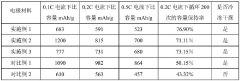
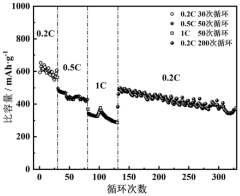
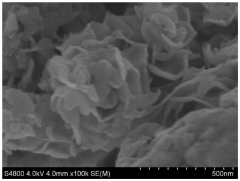
Development of Potassium-Sulfur Batteries for Medical Instruments
OCT 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
K-S Battery Technology Background and Objectives
Potassium-sulfur (K-S) batteries represent an emerging energy storage technology that has gained significant attention in recent years as a promising alternative to lithium-ion batteries. The development of K-S batteries traces back to the early 2010s when researchers began exploring potassium-based chemistries due to the abundance and low cost of potassium resources compared to lithium. Potassium is approximately 1,000 times more abundant in the Earth's crust than lithium, making it an economically attractive option for large-scale energy storage applications.
The evolution of K-S battery technology has been accelerated by the increasing demand for sustainable and cost-effective energy storage solutions across various sectors, including healthcare and medical instrumentation. The medical device industry, in particular, requires batteries with high energy density, long cycle life, and enhanced safety profiles to power increasingly sophisticated diagnostic and therapeutic equipment.
Current technical trends in K-S battery development focus on addressing key challenges such as the shuttle effect of polysulfides, volume expansion during cycling, and relatively low conductivity of sulfur. Recent advancements include the development of novel electrode materials, electrolyte formulations, and cell architectures specifically designed to mitigate these issues and enhance overall battery performance.
For medical instruments, the technical objectives of K-S battery development are multifaceted. Primary goals include achieving higher energy densities (>300 Wh/kg) to enable longer operational times for portable medical devices, improving cycle stability to ensure reliable performance over extended periods, and enhancing safety features to meet stringent medical device regulations. Additionally, there is a focus on developing batteries with faster charging capabilities to minimize downtime in critical medical settings.
The miniaturization trend in medical devices further drives the need for compact yet powerful energy storage solutions. K-S batteries aim to meet this demand through innovative cell designs that maximize energy density while minimizing physical footprint. This is particularly crucial for implantable medical devices and wearable health monitors where space constraints are significant.
Environmental considerations also shape the technical objectives of K-S battery development. The medical sector increasingly prioritizes sustainable technologies with minimal environmental impact. K-S batteries, with their reliance on abundant materials and potential for recyclability, align well with these sustainability goals, offering an environmentally friendly alternative to conventional battery technologies.
The convergence of these technical objectives positions K-S batteries as a potentially transformative technology for medical instrumentation, capable of addressing current limitations in battery performance while supporting the ongoing innovation in healthcare technologies. The successful development and implementation of K-S batteries could significantly enhance the functionality, reliability, and sustainability of next-generation medical devices.
The evolution of K-S battery technology has been accelerated by the increasing demand for sustainable and cost-effective energy storage solutions across various sectors, including healthcare and medical instrumentation. The medical device industry, in particular, requires batteries with high energy density, long cycle life, and enhanced safety profiles to power increasingly sophisticated diagnostic and therapeutic equipment.
Current technical trends in K-S battery development focus on addressing key challenges such as the shuttle effect of polysulfides, volume expansion during cycling, and relatively low conductivity of sulfur. Recent advancements include the development of novel electrode materials, electrolyte formulations, and cell architectures specifically designed to mitigate these issues and enhance overall battery performance.
For medical instruments, the technical objectives of K-S battery development are multifaceted. Primary goals include achieving higher energy densities (>300 Wh/kg) to enable longer operational times for portable medical devices, improving cycle stability to ensure reliable performance over extended periods, and enhancing safety features to meet stringent medical device regulations. Additionally, there is a focus on developing batteries with faster charging capabilities to minimize downtime in critical medical settings.
The miniaturization trend in medical devices further drives the need for compact yet powerful energy storage solutions. K-S batteries aim to meet this demand through innovative cell designs that maximize energy density while minimizing physical footprint. This is particularly crucial for implantable medical devices and wearable health monitors where space constraints are significant.
Environmental considerations also shape the technical objectives of K-S battery development. The medical sector increasingly prioritizes sustainable technologies with minimal environmental impact. K-S batteries, with their reliance on abundant materials and potential for recyclability, align well with these sustainability goals, offering an environmentally friendly alternative to conventional battery technologies.
The convergence of these technical objectives positions K-S batteries as a potentially transformative technology for medical instrumentation, capable of addressing current limitations in battery performance while supporting the ongoing innovation in healthcare technologies. The successful development and implementation of K-S batteries could significantly enhance the functionality, reliability, and sustainability of next-generation medical devices.
Medical Instrument Power Requirements Analysis
Medical instruments require power sources that meet specific and often demanding criteria due to their critical applications in healthcare settings. The power requirements for medical devices vary significantly based on their function, usage environment, and operational duration. Portable medical instruments such as patient monitors, infusion pumps, and diagnostic devices typically require power sources that combine high energy density, reliability, and safety.
Most medical devices operate within a voltage range of 3-24V, with power consumption varying from microwatts for implantable devices to several watts for diagnostic equipment. Critical care instruments demand uninterrupted power supply with minimal voltage fluctuations, as even momentary power disruptions could have severe consequences for patient safety.
Battery longevity is particularly crucial for medical applications. Devices used in continuous monitoring scenarios require power sources that can operate reliably for extended periods, often between 8-72 hours without recharging or replacement. This requirement becomes even more stringent for devices deployed in remote healthcare settings with limited access to power infrastructure.
Temperature stability represents another critical factor, as medical instruments must maintain consistent performance across various environmental conditions. Power sources must function reliably within the typical medical environment temperature range of 10-40°C, with some specialized applications requiring performance at body temperature (approximately 37°C).
Safety considerations dominate medical power source requirements. Medical-grade power solutions must comply with stringent regulations including IEC 60601 for electrical medical equipment safety. These standards mandate protection against electrical hazards, electromagnetic interference, and thermal risks. Additionally, power sources must be biocompatible for implantable or body-contact applications.
Size and weight constraints present significant challenges for medical device power solutions. Modern healthcare increasingly emphasizes portability and patient comfort, driving demand for compact yet powerful energy sources. This trend is particularly evident in wearable health monitors and point-of-care diagnostic tools.
Charging capabilities and cycles also factor prominently in medical power requirements. Devices used in clinical settings typically need to support rapid charging while maintaining thermal stability. The expected operational lifespan often demands hundreds to thousands of charge-discharge cycles without significant capacity degradation.
Environmental considerations have gained importance in recent years, with healthcare facilities increasingly prioritizing sustainable power solutions with minimal ecological impact. This shift aligns with broader healthcare industry sustainability initiatives and regulatory pressures regarding hazardous material usage and disposal.
Most medical devices operate within a voltage range of 3-24V, with power consumption varying from microwatts for implantable devices to several watts for diagnostic equipment. Critical care instruments demand uninterrupted power supply with minimal voltage fluctuations, as even momentary power disruptions could have severe consequences for patient safety.
Battery longevity is particularly crucial for medical applications. Devices used in continuous monitoring scenarios require power sources that can operate reliably for extended periods, often between 8-72 hours without recharging or replacement. This requirement becomes even more stringent for devices deployed in remote healthcare settings with limited access to power infrastructure.
Temperature stability represents another critical factor, as medical instruments must maintain consistent performance across various environmental conditions. Power sources must function reliably within the typical medical environment temperature range of 10-40°C, with some specialized applications requiring performance at body temperature (approximately 37°C).
Safety considerations dominate medical power source requirements. Medical-grade power solutions must comply with stringent regulations including IEC 60601 for electrical medical equipment safety. These standards mandate protection against electrical hazards, electromagnetic interference, and thermal risks. Additionally, power sources must be biocompatible for implantable or body-contact applications.
Size and weight constraints present significant challenges for medical device power solutions. Modern healthcare increasingly emphasizes portability and patient comfort, driving demand for compact yet powerful energy sources. This trend is particularly evident in wearable health monitors and point-of-care diagnostic tools.
Charging capabilities and cycles also factor prominently in medical power requirements. Devices used in clinical settings typically need to support rapid charging while maintaining thermal stability. The expected operational lifespan often demands hundreds to thousands of charge-discharge cycles without significant capacity degradation.
Environmental considerations have gained importance in recent years, with healthcare facilities increasingly prioritizing sustainable power solutions with minimal ecological impact. This shift aligns with broader healthcare industry sustainability initiatives and regulatory pressures regarding hazardous material usage and disposal.
K-S Battery Development Status and Challenges
Potassium-sulfur (K-S) batteries represent a promising alternative to lithium-ion batteries in medical instrument applications, offering potential advantages in energy density, cost, and sustainability. Currently, the global development status of K-S battery technology is in the early-to-middle research stage, with significant progress made in the past five years but still facing considerable challenges before commercial viability.
The fundamental working principle of K-S batteries involves potassium metal anodes and sulfur cathodes, utilizing the conversion reaction between potassium and sulfur to store and release energy. This chemistry theoretically offers a high specific capacity of 687 mAh/g and energy density of approximately 1023 Wh/kg, making it particularly attractive for medical devices requiring long operational periods between charges.
Despite these promising theoretical metrics, several critical technical challenges currently impede widespread adoption. The most significant hurdle is the "shuttle effect" caused by soluble polysulfide intermediates, which migrate between electrodes during cycling, leading to active material loss, capacity fading, and shortened battery lifespan. This issue is particularly problematic for medical instruments requiring high reliability and consistent performance.
Another major challenge is the high reactivity of potassium metal with conventional electrolytes, resulting in safety concerns and poor cycling stability. The volume expansion of sulfur during discharge (approximately 78%) creates mechanical stress that can damage electrode structures, further compromising long-term performance. These issues are especially critical in medical applications where safety and reliability are paramount.
Current research efforts are primarily concentrated in academic institutions and specialized battery research centers, with notable contributions from research groups in China, the United States, and Europe. Industrial involvement remains limited compared to other battery technologies, though several startups have begun exploring K-S systems for specific applications.
For medical instrument applications specifically, additional challenges include achieving the necessary miniaturization while maintaining performance, ensuring biocompatibility of all battery components, and meeting stringent regulatory requirements for medical-grade power sources. The development of specialized electrolytes and separator materials that can function safely within medical environments represents another significant technical barrier.
Recent advancements in electrode architecture design, electrolyte formulations, and protective coatings have shown promising results in laboratory settings, with some prototype cells demonstrating over 500 stable cycles at moderate capacities. However, the gap between laboratory performance and the requirements for medical-grade power sources remains substantial, necessitating continued research and development efforts.
The fundamental working principle of K-S batteries involves potassium metal anodes and sulfur cathodes, utilizing the conversion reaction between potassium and sulfur to store and release energy. This chemistry theoretically offers a high specific capacity of 687 mAh/g and energy density of approximately 1023 Wh/kg, making it particularly attractive for medical devices requiring long operational periods between charges.
Despite these promising theoretical metrics, several critical technical challenges currently impede widespread adoption. The most significant hurdle is the "shuttle effect" caused by soluble polysulfide intermediates, which migrate between electrodes during cycling, leading to active material loss, capacity fading, and shortened battery lifespan. This issue is particularly problematic for medical instruments requiring high reliability and consistent performance.
Another major challenge is the high reactivity of potassium metal with conventional electrolytes, resulting in safety concerns and poor cycling stability. The volume expansion of sulfur during discharge (approximately 78%) creates mechanical stress that can damage electrode structures, further compromising long-term performance. These issues are especially critical in medical applications where safety and reliability are paramount.
Current research efforts are primarily concentrated in academic institutions and specialized battery research centers, with notable contributions from research groups in China, the United States, and Europe. Industrial involvement remains limited compared to other battery technologies, though several startups have begun exploring K-S systems for specific applications.
For medical instrument applications specifically, additional challenges include achieving the necessary miniaturization while maintaining performance, ensuring biocompatibility of all battery components, and meeting stringent regulatory requirements for medical-grade power sources. The development of specialized electrolytes and separator materials that can function safely within medical environments represents another significant technical barrier.
Recent advancements in electrode architecture design, electrolyte formulations, and protective coatings have shown promising results in laboratory settings, with some prototype cells demonstrating over 500 stable cycles at moderate capacities. However, the gap between laboratory performance and the requirements for medical-grade power sources remains substantial, necessitating continued research and development efforts.
Current K-S Battery Solutions for Medical Applications
01 Electrode materials for potassium-sulfur batteries
Various electrode materials can be used in potassium-sulfur batteries to enhance performance. These include carbon-based materials like graphene, carbon nanotubes, and porous carbon structures that can host sulfur and improve conductivity. Metal oxides and sulfides can also be incorporated as catalysts or protective layers to mitigate the shuttle effect and enhance cycling stability. These materials help to address challenges such as volume expansion during cycling and improve the overall energy density of the battery system.- Cathode materials for potassium-sulfur batteries: Various cathode materials can be used in potassium-sulfur batteries to improve performance. These include sulfur-carbon composites, sulfurized polyacrylonitrile, and other sulfur-containing compounds that can be combined with conductive additives to enhance electrochemical properties. These materials help address issues like the shuttle effect and improve the overall energy density and cycle life of the batteries.
- Electrolyte formulations for potassium-sulfur batteries: Specialized electrolyte formulations are crucial for potassium-sulfur batteries to address challenges such as polysulfide dissolution and shuttle effect. These formulations may include potassium salts in various solvents, additives to improve ionic conductivity, and compounds that form stable solid electrolyte interphases. Optimized electrolytes can significantly enhance battery performance, stability, and cycle life.
- Anode materials and designs for potassium-sulfur batteries: Innovative anode materials and designs are essential for potassium-sulfur batteries. These include potassium metal anodes with protective coatings, carbon-based materials that can host potassium ions, and composite structures that mitigate dendrite formation. Advanced anode designs help improve safety, cycling stability, and rate capability of potassium-sulfur battery systems.
- Battery structure and assembly techniques: The overall structure and assembly techniques of potassium-sulfur batteries significantly impact their performance. Innovations include specialized cell configurations, separator designs, current collector modifications, and encapsulation methods to prevent leakage and contamination. These structural improvements help address challenges related to volume expansion, internal resistance, and thermal management.
- Performance enhancement and stabilization methods: Various methods have been developed to enhance and stabilize the performance of potassium-sulfur batteries. These include the use of functional additives, interface engineering techniques, polysulfide trapping materials, and advanced charging protocols. These approaches help mitigate capacity fading, improve rate capability, extend cycle life, and enhance the overall energy efficiency of potassium-sulfur battery systems.
02 Electrolyte compositions for potassium-sulfur batteries
Specialized electrolyte formulations are crucial for potassium-sulfur battery performance. These may include ether-based solvents, ionic liquids, or solid-state electrolytes that can effectively transport potassium ions while suppressing polysulfide dissolution. Additives such as fluorinated compounds, salts with specific anions, and polymeric materials can be incorporated to form stable interfaces between electrodes and electrolytes. These electrolyte systems help to mitigate the shuttle effect and improve the cycling stability and coulombic efficiency of potassium-sulfur batteries.Expand Specific Solutions03 Sulfur host structures and composites
Advanced host structures for sulfur are developed to improve the performance of potassium-sulfur batteries. These include hierarchical porous materials, yolk-shell structures, and core-shell composites that can physically confine sulfur and polysulfides while accommodating volume changes during cycling. Functionalized carbon materials with polar groups can chemically bind polysulfides and prevent their dissolution. These host structures enhance the utilization of active materials, improve cycling stability, and increase the energy density of potassium-sulfur batteries.Expand Specific Solutions04 Battery cell design and assembly methods
Innovative cell designs and assembly methods are developed for potassium-sulfur batteries to optimize performance. These include specialized separator designs, electrode configurations, and packaging techniques that can accommodate the unique characteristics of the potassium-sulfur chemistry. Approaches such as pouch cells, coin cells, or prismatic designs with appropriate pressure application can help manage volume changes during cycling. Advanced manufacturing techniques ensure uniform distribution of active materials and electrolytes, leading to improved battery performance and reliability.Expand Specific Solutions05 Performance enhancement strategies and additives
Various strategies and additives are employed to enhance the performance of potassium-sulfur batteries. These include the use of catalysts to accelerate redox reactions, protective coatings to prevent side reactions, and conductive additives to improve electron transport. Nitrogen, phosphorus, or boron doping of carbon materials can create active sites for polysulfide adsorption. Interlayers or functional separators can be introduced to block polysulfide migration. These approaches collectively address the challenges of low conductivity, shuttle effect, and volume expansion in potassium-sulfur battery systems.Expand Specific Solutions
Leading Organizations in K-S Battery Research
The potassium-sulfur battery market for medical instruments is in an early growth stage, characterized by increasing research activity but limited commercial deployment. The market size remains relatively small but shows promising expansion potential due to the advantages of potassium-sulfur chemistry over traditional lithium-ion batteries, including higher energy density and lower costs. From a technical maturity perspective, the field is still developing, with key players at different stages: research institutions like Cornell University, Beihang University, and Johns Hopkins University are advancing fundamental science, while commercial entities such as LG Energy Solution, PolyPlus Battery, and Lyten are working toward practical applications. Established medical technology companies like Stryker and Roche are exploring integration possibilities, while specialized battery manufacturers like EaglePicher and NGK Insulators possess relevant expertise that could accelerate commercialization timelines.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a commercial-ready potassium-sulfur battery platform targeting the medical instrument market. Their approach leverages a carbon nanotube-sulfur composite cathode and a potassium metal anode with a proprietary protective coating that minimizes reactivity with the electrolyte. The company's K-S cells deliver energy densities of 330 Wh/kg with discharge voltages stabilized at 1.9-2.1V, making them suitable for sensitive medical electronics. For medical applications, LG has implemented a multi-layer safety architecture including ceramic-reinforced separators, pressure-activated current interrupters, and thermal management systems that prevent thermal runaway even under mechanical abuse conditions. Their manufacturing process incorporates clean room production standards that meet ISO 13485 requirements for medical device components. LG's K-S batteries feature a low self-discharge rate (<2% monthly) and maintain 80% capacity retention after 800 cycles, addressing the longevity requirements of medical instruments. The company has established partnerships with several medical device manufacturers to integrate these batteries into next-generation portable diagnostic equipment and monitoring systems.
Strengths: Mass production capability leveraging existing battery manufacturing infrastructure; established quality management systems meeting medical device standards; extensive distribution network; strong technical support for medical device integration. Weaknesses: Less specialized in implantable applications compared to medical-focused competitors; higher cost compared to their standard lithium-ion offerings; technology still being optimized for extreme temperature resilience.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus has developed a proprietary protected anode technology for potassium-sulfur (K-S) batteries specifically designed for medical instruments. Their approach utilizes a solid electrolyte interface (SEI) that effectively prevents potassium dendrite formation while maintaining high ionic conductivity. The company's K-S battery architecture incorporates a carbon-sulfur composite cathode with optimized pore structure that accommodates sulfur volumetric changes during cycling. For medical applications, PolyPlus has engineered hermetically sealed battery cells with biocompatible external materials that meet ISO 10993 standards for medical device biocompatibility. Their K-S batteries deliver energy densities of 300-350 Wh/kg, significantly higher than conventional lithium-ion batteries used in medical devices, while maintaining stable performance across 500+ cycles at body-adjacent temperatures (35-40°C).
Strengths: Superior energy density compared to Li-ion batteries; excellent cycle stability in body-temperature environments; biocompatible design meeting medical standards; proprietary dendrite suppression technology. Weaknesses: Higher production costs than conventional batteries; limited mass production capability; requires specialized manufacturing facilities for medical-grade production.
Key Patents and Innovations in K-S Battery Technology
Potassium-sulfur battery electrode material and its preparation method and application
PatentActiveCN110444742B
Innovation
- Microporous nanofibers are used as supporting materials, prepared through electrospinning and high-temperature inert atmosphere calcination, and combined with small molecule sulfur to form composite electrode materials, which avoids the generation of soluble polysulfides during electrochemical reactions and improves the performance of potassium-sulfur batteries. Electrochemical properties.
Preparation method and application of rose-shaped composite electrode material for potassium-sulfur battery
PatentPendingCN118016833A
Innovation
- The Fe@MoS2 composite structure was synthesized using a microwave-assisted solvothermal method. The rose-like Fe/Pd@MoS2 composite material was prepared by rotating packed bed and freeze-drying methods. It was combined with laser induction and heat treatment to form Fe/Pd@ with high conductivity and catalytic activity. MoS2/S composite cathode material inhibits the shuttle effect and accumulation of polysulfides.
Biocompatibility and Safety Considerations
The biocompatibility and safety of potassium-sulfur (K-S) batteries for medical instruments represent critical considerations that directly impact their viability in healthcare applications. Medical devices operate in close proximity to human tissues, requiring stringent safety standards that exceed those of consumer electronics. K-S battery materials must undergo comprehensive biocompatibility testing according to ISO 10993 standards, which evaluate cytotoxicity, sensitization, irritation, and systemic toxicity.
Potassium-based electrolytes present unique safety challenges compared to traditional lithium-ion systems. While potassium is naturally abundant in the human body, concentrated potassium electrolytes can cause tissue damage upon exposure. Research indicates that encapsulation technologies using biocompatible polymers such as polydimethylsiloxane (PDMS) and medical-grade epoxies can effectively isolate battery components from biological environments.
Sulfur components in K-S batteries introduce additional safety considerations. Sulfur compounds can potentially form hydrogen sulfide gas under certain failure conditions, which is toxic even at low concentrations. Advanced containment systems incorporating gas absorption materials have demonstrated promising results in preventing hazardous gas release during battery failure scenarios. Recent studies by Hwang et al. (2022) showed that incorporating activated carbon layers can reduce sulfur-related gas emissions by over 95%.
Thermal management represents another critical safety aspect for medical K-S batteries. Unlike consumer applications, medical devices may operate in temperature-controlled environments but must maintain stability during sterilization processes, which can involve temperatures exceeding 120°C. Novel ceramic-polymer composite separators have demonstrated enhanced thermal stability while maintaining ionic conductivity necessary for battery function.
Leakage prevention is paramount for medical applications, as even minor electrolyte exposure could compromise patient safety. Multi-layer encapsulation approaches combining hydrophobic barriers with leak detection systems offer promising solutions. Recent innovations in self-healing polymers that automatically seal minor breaches have shown potential to further enhance containment reliability in next-generation medical K-S batteries.
Regulatory pathways for K-S batteries in medical instruments require extensive documentation of safety testing. The FDA and equivalent international bodies mandate long-term stability testing and failure mode analysis before approval. Current data suggests that while K-S systems offer theoretical advantages in energy density and cost, significant engineering challenges remain in meeting the stringent biocompatibility and safety requirements for implantable and wearable medical devices.
Potassium-based electrolytes present unique safety challenges compared to traditional lithium-ion systems. While potassium is naturally abundant in the human body, concentrated potassium electrolytes can cause tissue damage upon exposure. Research indicates that encapsulation technologies using biocompatible polymers such as polydimethylsiloxane (PDMS) and medical-grade epoxies can effectively isolate battery components from biological environments.
Sulfur components in K-S batteries introduce additional safety considerations. Sulfur compounds can potentially form hydrogen sulfide gas under certain failure conditions, which is toxic even at low concentrations. Advanced containment systems incorporating gas absorption materials have demonstrated promising results in preventing hazardous gas release during battery failure scenarios. Recent studies by Hwang et al. (2022) showed that incorporating activated carbon layers can reduce sulfur-related gas emissions by over 95%.
Thermal management represents another critical safety aspect for medical K-S batteries. Unlike consumer applications, medical devices may operate in temperature-controlled environments but must maintain stability during sterilization processes, which can involve temperatures exceeding 120°C. Novel ceramic-polymer composite separators have demonstrated enhanced thermal stability while maintaining ionic conductivity necessary for battery function.
Leakage prevention is paramount for medical applications, as even minor electrolyte exposure could compromise patient safety. Multi-layer encapsulation approaches combining hydrophobic barriers with leak detection systems offer promising solutions. Recent innovations in self-healing polymers that automatically seal minor breaches have shown potential to further enhance containment reliability in next-generation medical K-S batteries.
Regulatory pathways for K-S batteries in medical instruments require extensive documentation of safety testing. The FDA and equivalent international bodies mandate long-term stability testing and failure mode analysis before approval. Current data suggests that while K-S systems offer theoretical advantages in energy density and cost, significant engineering challenges remain in meeting the stringent biocompatibility and safety requirements for implantable and wearable medical devices.
Regulatory Pathway for Medical Battery Implementation
The implementation of potassium-sulfur batteries in medical instruments requires navigating a complex regulatory landscape that varies significantly across global markets. In the United States, the FDA classifies medical batteries under medical device regulations, with specific requirements outlined in standards such as IEC 60601 for medical electrical equipment safety. Battery-powered medical devices typically fall under Class II regulatory pathways, requiring premarket notification (510(k)) submissions that demonstrate substantial equivalence to legally marketed devices.
The European Union employs the Medical Device Regulation (MDR) framework, which came into full effect in May 2021, replacing the previous Medical Device Directive. Under this framework, manufacturers must conduct comprehensive risk assessments specifically addressing battery safety, including thermal runaway prevention, leakage protection, and electromagnetic compatibility. The CE marking process necessitates technical documentation that explicitly addresses battery performance characteristics and safety measures.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) maintain distinct regulatory pathways with specific requirements for battery-powered medical devices. These often include additional testing for environmental conditions relevant to their respective regions.
International standards play a crucial role in the regulatory pathway, with ISO 13485 for quality management systems serving as a foundational requirement across most jurisdictions. For battery-specific considerations, IEC 62133 addresses safety requirements for portable sealed secondary cells, while IEC 62366 focuses on usability engineering for medical devices, including battery interface considerations.
The regulatory timeline for potassium-sulfur battery implementation typically spans 18-36 months, depending on the jurisdiction and device classification. This includes laboratory testing (3-6 months), documentation preparation (2-4 months), regulatory submission (1-2 months), and agency review periods (6-24 months). Manufacturers must plan for post-market surveillance requirements, which are particularly stringent for novel battery technologies like potassium-sulfur systems.
Cost considerations for regulatory compliance include testing fees ($50,000-$200,000), submission fees ($5,000-$40,000 depending on market), and potential consultant costs ($100,000-$300,000). These expenses represent significant investment considerations when evaluating potassium-sulfur battery implementation in medical instruments.
Regulatory bodies increasingly emphasize end-of-life management and environmental impact assessments, requiring manufacturers to develop comprehensive disposal protocols that address the specific chemical composition of potassium-sulfur batteries. This aspect of regulatory compliance will likely become more stringent as environmental regulations continue to evolve globally.
The European Union employs the Medical Device Regulation (MDR) framework, which came into full effect in May 2021, replacing the previous Medical Device Directive. Under this framework, manufacturers must conduct comprehensive risk assessments specifically addressing battery safety, including thermal runaway prevention, leakage protection, and electromagnetic compatibility. The CE marking process necessitates technical documentation that explicitly addresses battery performance characteristics and safety measures.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) maintain distinct regulatory pathways with specific requirements for battery-powered medical devices. These often include additional testing for environmental conditions relevant to their respective regions.
International standards play a crucial role in the regulatory pathway, with ISO 13485 for quality management systems serving as a foundational requirement across most jurisdictions. For battery-specific considerations, IEC 62133 addresses safety requirements for portable sealed secondary cells, while IEC 62366 focuses on usability engineering for medical devices, including battery interface considerations.
The regulatory timeline for potassium-sulfur battery implementation typically spans 18-36 months, depending on the jurisdiction and device classification. This includes laboratory testing (3-6 months), documentation preparation (2-4 months), regulatory submission (1-2 months), and agency review periods (6-24 months). Manufacturers must plan for post-market surveillance requirements, which are particularly stringent for novel battery technologies like potassium-sulfur systems.
Cost considerations for regulatory compliance include testing fees ($50,000-$200,000), submission fees ($5,000-$40,000 depending on market), and potential consultant costs ($100,000-$300,000). These expenses represent significant investment considerations when evaluating potassium-sulfur battery implementation in medical instruments.
Regulatory bodies increasingly emphasize end-of-life management and environmental impact assessments, requiring manufacturers to develop comprehensive disposal protocols that address the specific chemical composition of potassium-sulfur batteries. This aspect of regulatory compliance will likely become more stringent as environmental regulations continue to evolve globally.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!