Spin trap nitronyl hindered phenols
a technology of nitronyl and spin trap, which is applied in the field ofnitronyl substituted phenols, can solve the problem that molecules have not been availabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Lipid peroxidation (LPO) initiated by tert-butoxy radicals
Male Sprague-Dawley rats (175-200 g) that had been maintained on commercial rat chow were starved for 24 hours, sacrificed and the livers removed. Microsomes were prepared by homogenizing the minced livers in 0.15 M potassium phosphate buffer pH 7.4 5 ml / g) and then centrifuging at 12,000.times.g for 15 minutes. The supernatant fraction was then centrifuged at 105,000.times.g for 90 minutes. The microsomal pellets were washed twice by resuspending in buffer and centrifuging at 105,000.times.g for 60 minutes. The microsomal pellets were kept frozen at -80.degree. C. until ready for use. Rat liver microsome pellets were made up into microsomal suspension (MS) by adding one milliliter of 50 mM potassium phosphate buffer (pH 7.4) per gram of liver. The protein concentration of microsomal suspensions were determined by the method of O. H. Lowry et al., J. Biol. Chem. 193:265-75 (1951).
Reaction samples were 1 ml (milliliter) in vol...
example 2
LPO initiated by NADPH catalyzed cytochrome-P450 metabolism of CCl.sub.4
An amount equal to 0.09 mmole spin trap was weighed into a test-tube to give a final concentration of 30 mM. To this container was added 14 .mu.l .sup.13 CCL.sub.4 (to produce a final concentration of 50 mM). After shaking to dissolve the spin trap, 2.35 ml of 50 mM potassium phosphate buffer at pH 7.4 (KH.sub.2 PO.sup.4 and K.sub.2 HPO.sub.4) was added plus 0.3 ml microsomal suspension. The solution was vortexed and preincubated for 10 minutes at room temperature (25.degree. C.). At this point, 0.3 ml NADPH generating system (0.5 Kornberg units of glucose-6-phosphate dehydrogenase, 5 .mu.M glucose-6-phosphate and 0.3 mM NADPH) was added to start the reaction. After incubation for 15 min at room temperature, 1.0 ml toluene was added and the system vortexed. Following centrifugation for 30 minutes at 3000 rpm (Sorvell GLC-4 with swinging bucket rotor H1000) approximately 500 .mu.l supernatant solution is removed ...
example 3
Rat Brain Miocrosomes
Similar tests were performed with rat brain microsomes using the TBA test for MDA production as the result of LPO. Results on the same compounds were as follows:
______________________________________ Inhibitor Concentration for 50% Inhibition (IC.sub.50) ______________________________________ BHT (not available) PBN 1000 .mu.M DBBNP 0.7 .mu.M DBONP 0.3 .mu.M DMONP 70 .mu.M DMBNP 600 .mu.M ON 76 .mu.M ______________________________________
Although BHT was not available for this test it is not believed that it would be particularly effective with this particular system.
The data obtained demonstrates that DBBNP and DBONP are very good inhibitors in these systems.
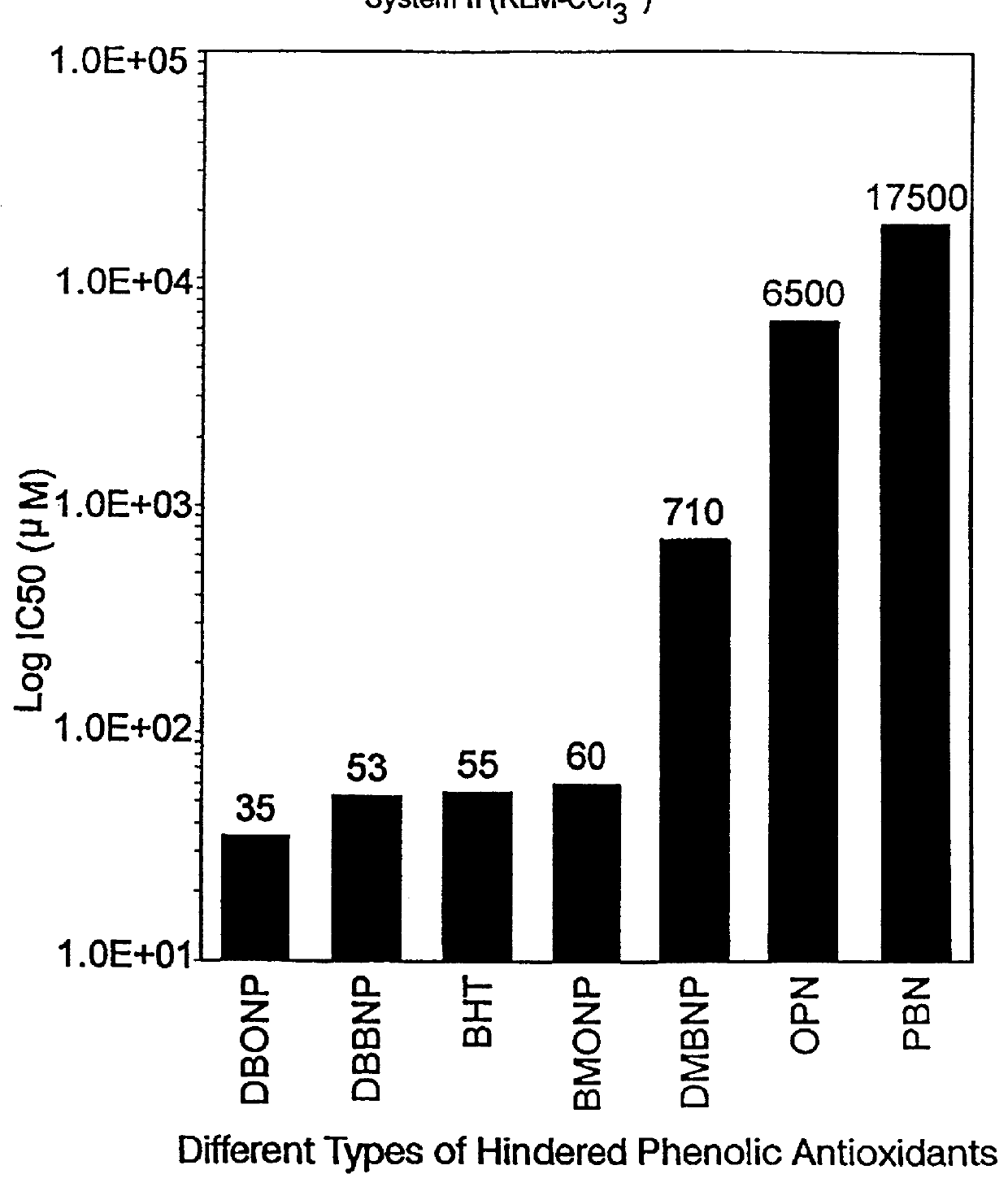
______________________________________ IC.sub.50 RLM (I) RLM (II) RBM (III) ______________________________________ DBBNP 4.3 .mu.M 53 .mu.M 0.7 .mu.M (700 nM) DBONP 5.8 .mu.M 35 .mu.M 0.3 .mu.M (300 mM) ______________________________________
The above data in Examples 1, 2 and 3 are graphically represented, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Body weight | aaaaa | aaaaa |
| Inhibitor concentration | aaaaa | aaaaa |
| Inhibitor concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com