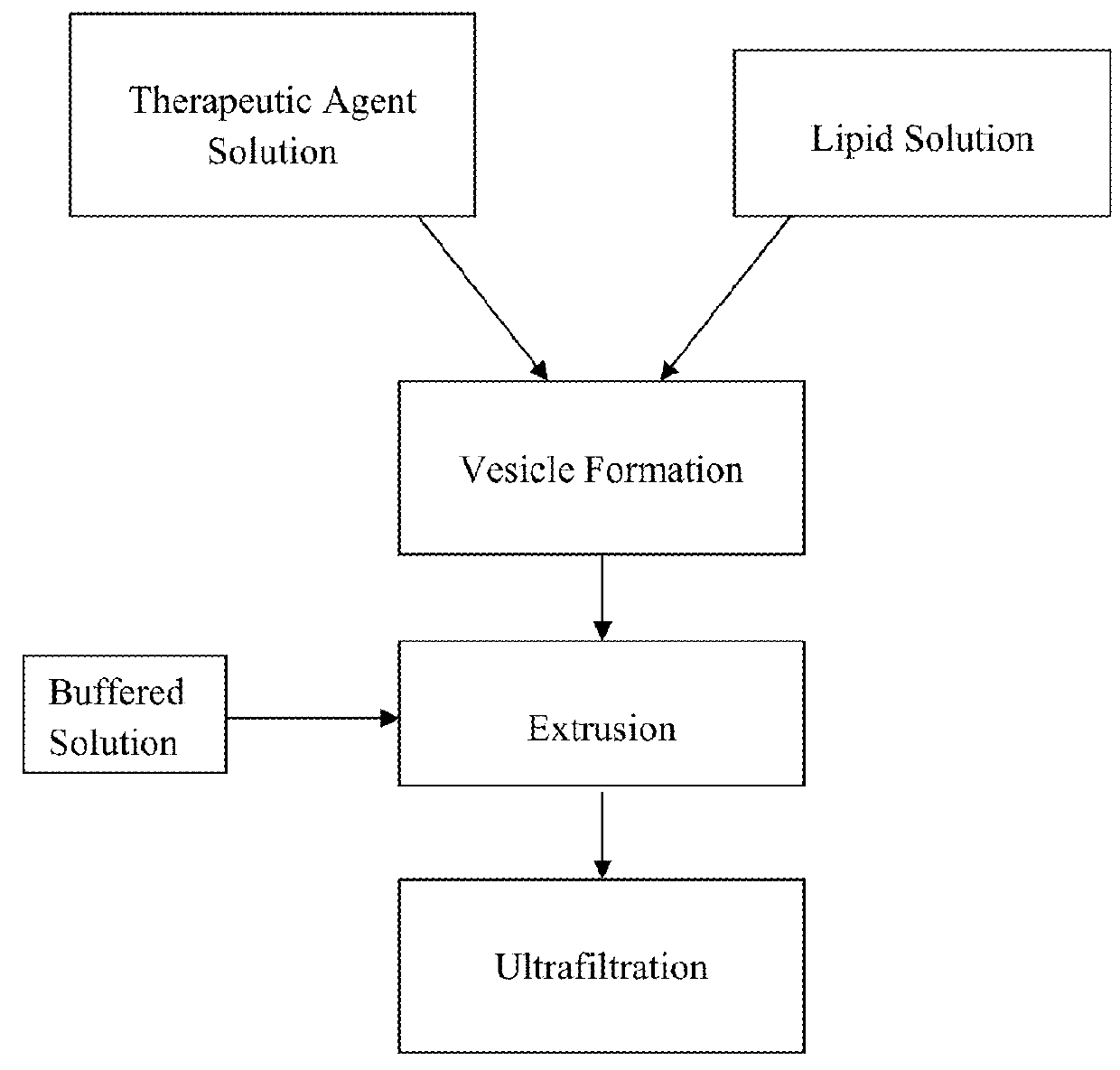
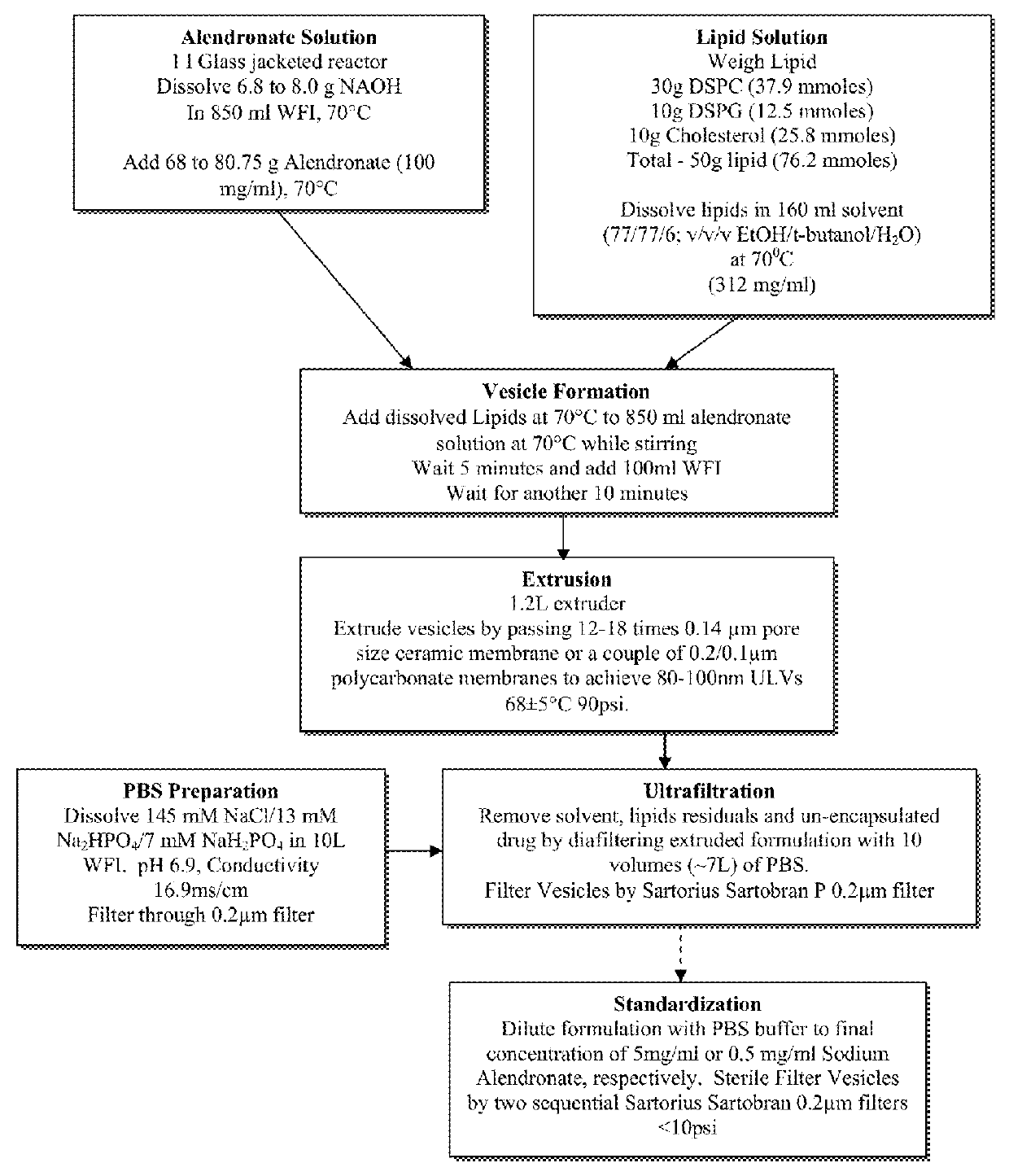
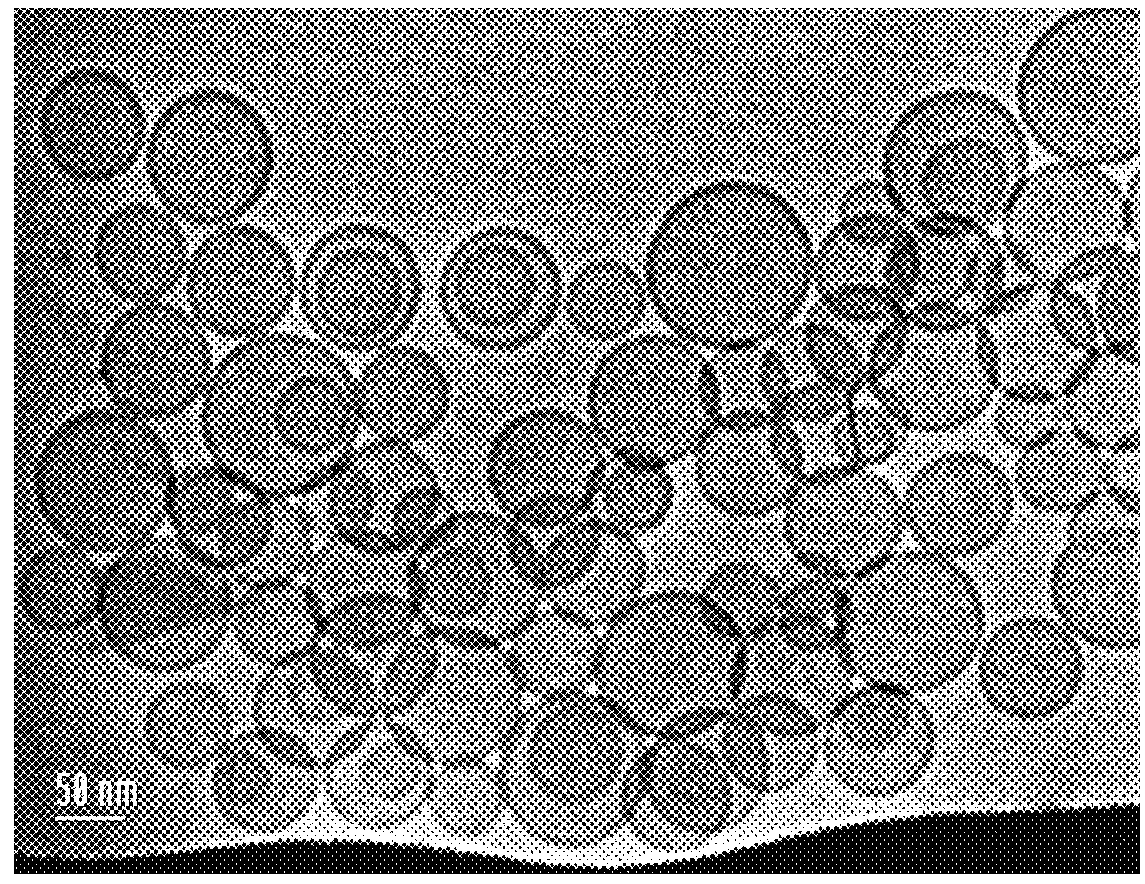
Liposome formulation and manufacture
a technology of liposome and liposome, which is applied in the direction of liposomal delivery, medical preparations, pharmaceutical non-active ingredients, etc., can solve the problems of high large operating cost and time, and the size and shape of liposomal formulations known in the art are not substantially uniform, so as to reduce side effects, increase the degree of confidence in sterilization process, and the effect of size uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation Batch Preparation
[0066]In accordance with the process described above, an example batch was prepared. Clearly the batch size may be varied, as desired for commercial production. In this example, a one liter batch of liposomal alendronate encapsulated in liposomes containing cholesterol, DSPC and DSPG, and dispersed in phosphate buffer saline solution was produced. Liposomal alendronate may be provided in two concentrations, for clinical convenience: 5 mg / ml and 0.5 mg / ml, as a sterile whitish, liposomal dispersion. These concentrations may be further formulated to obtain a desired amount of therapeutic agent in any specific volume. The lipid ingredients were composed of cholesterol, DSPC and DSPG. The dispersion also contained a phosphate buffer saline solution for pH control, infusion suitability and for the maintenance of isotonicity. At least 96% of the drug in the final product was encapsulated in the liposomes. For administration, the content of the vial (or part of...
example 2
Rigidity Testing
[0091]Four samples of large unilamellar liposomes (diameter approx. 100 nm) were analyzed for rigidity. Empty liposomes dissolved in PBS made in accordance with the present invention were labeled LPO. Liposome formulations containing 5.0 mg / ml of alendronate made in accordance with the present invention were labeled as LSA. Two other liposome samples, labeled KS and HU, dissolved in HEPES, were made in accordance with the prior art method described in Epstein-Barash, Hila, et al., “Physicochemical parameters affecting liposomal bisphosphonates bioactivity for restenosis therapy: Internalization, cell inhibition, activation of cytokines and complement, and mechanism of cell death”, J. Controlled Release 146 (2010) 182-195
[0092]Each sample was analyzed for specific volume compressibility of liposomes. Using ultrasound velocimetry, the elastic properties of the liposomes was evaluated based on the following relationship:
[0093]βS=1ρ·u2,(1)
where βS, ρ, and u are the adiab...
example 3
Stability Analysis
[0103]The stability of the liposome formulation made in accordance with Example 1 is exemplified in Tables 4 and 5. Table 4 demonstrates that the liposomes of the current invention are stable when stored at 4 degrees C. at least through 36 months, and the formulation meets all required specifications.
[0104]
TABLE 4Stability at 4 C.SpecBase1 Mo7 Mo12 Mo24 Mo36 MoappearancewhitishconformconformconformconformconformconformdispersionAlendronate0.5 ± 0.050.490.530.540.510.520.52(mg / ml)Encapsulation>9698%98%96>98%>99%>99%Percentage (%)DSPC (mg / ml)1.4-2.01.91.821.61.81.8DSPG (mg / ml)0.5-0.70.60.60.60.50.60.6Chol. (mg / ml)0.5-0.70.550.590.560.580.60.61drug:lipid ratio1:5.7 ± 1.0 1;6.21;5.61;5.91;5.31;5.81;5.8vesicles sizediameter92 nm92 nm92 nm92 nm91 nm91 nm(nm)100 ± 30 pH6.7-7.36.96.96.9776.9osmolality270-340309na317316312312(mo / kg)
[0105]Table 5 demonstrates that the liposomes of the current invention are stable when stored at 25 degrees C. at least through 7 months, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com