Sperm specific proteins
a technology of specific proteins and sperm, which is applied in the field of sperm specific proteins, can solve the problems of increasing the incidence of urogenital infections and cervicovaginal inflammation, affecting the safety of sperm, and affecting the safety of sperm, and achieves the effect of identifying a human sperm antigen that functions as a contraceptive vaccine with a level of efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Characterization of C7 / 8 2-D Electrophoresis, Standard Protein Gels and Western Blotting
[0102]Human sperm proteins for 2-D gel electrophoresis were solubilized and separated as previously described by Naaby-Hansen et al. (1997). Briefly, fresh semen specimens were allowed to liquify for up to 3 h before centrifugation on two-layer (80% and 55% in Ham s F-10 medium) Percoll density gradients. After subsequent washing of the sperm pellet collected from the bottom of the 80% gradient 3 times in Ham s F-10 medium, the sperm were solubilized in a lysis buffer consisting of 2% (v:v) NP-40, 9.8 M urea, 100 mM DTT, 2% ampholines (pH 3.5-10) and a cocktail of protease inhibitors before performing isoelectric focusing on 0.15 mg of sperm proteins in a 15×0.15 cm acrylamide rod containing a carrier ampholine (Pharmacia) composition of 28% pH 3.5-5, 20% pH 5-7, 7% pH 7-9 and 45% pH 3.5-10 in a gel composition described previously (Naaby-Hansen et al., 1997). Prior to electrophores...
example 2
Isolation of SAMP32
[0119]TritonX-114 Phase Partitioning and Two-dimensional Gel Electrophresis and Vectorial Labeling by Biotin
[0120]Human semen samples were obtained from healthy donor(s) as described (Mandal et. al., 1999). Triton X-114 phase partitioning was done according to Bordier (Bordier, 1981). Two-dimensional gel electrophresis was conducted as described in Example 1. Proteins were biotin labeled using standard techniques.
[0121]SDS-PAGE and Western Blot
[0122]Proteins were separated first on discontinuous polyacrylamide gel (4% stacking and 15% separating gel) at 100 volts voltage constant on a Bio-Rad mini-gel apparatus (Bio-Rad, California). Gels were then either stained with Coommassie blue or used for transfer to the membrane in western blot. For the western blot, proteins were transferred to Nitrocellulose membrane (0.2 um) at 100 volts voltage constant for one hour with cooling. At completion, membranes were blocked in PBST containing 5% non-fat milk, and 1% normal go...
example 3
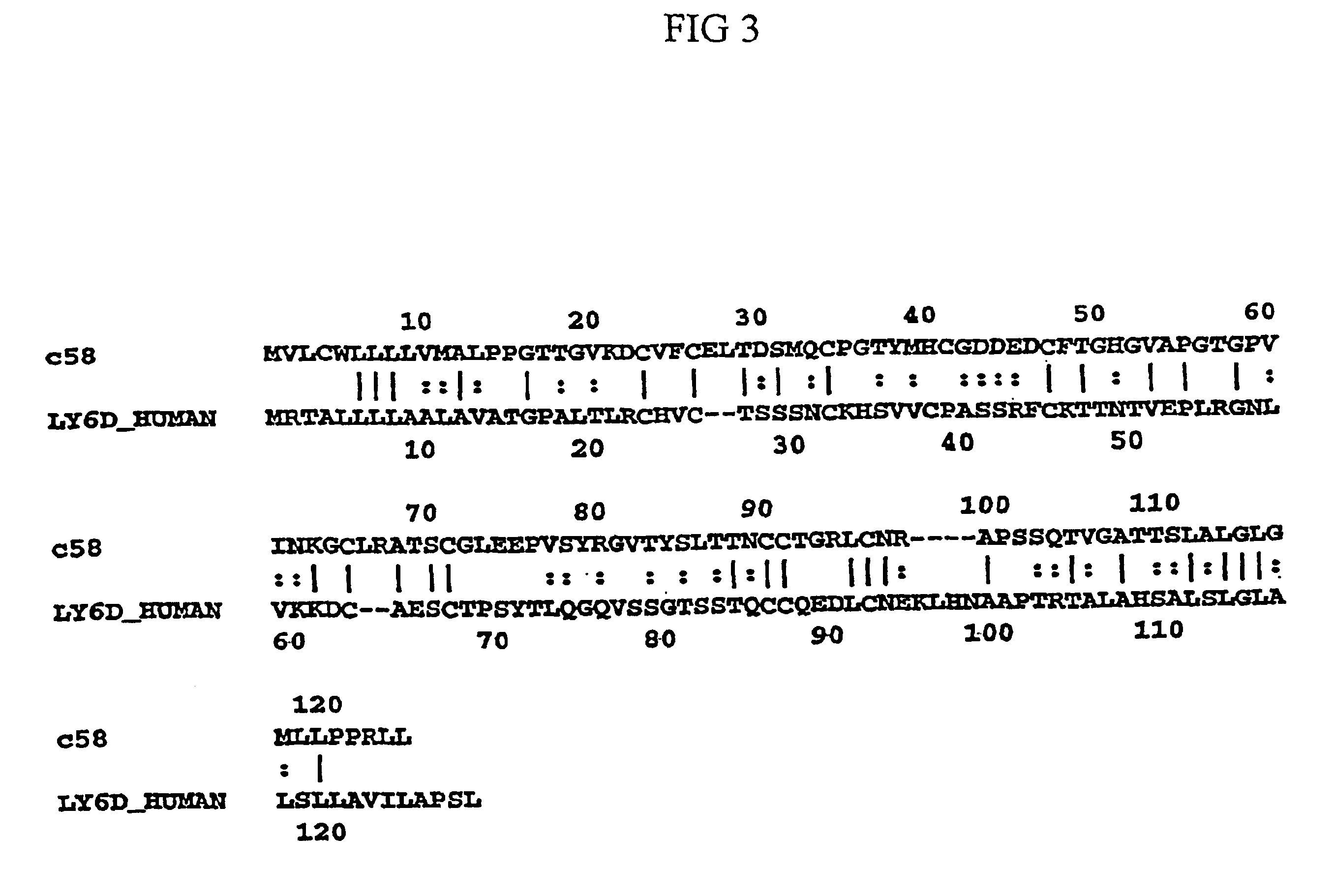
Isolation of C58
[0140]Separation of Hydrophobic, Putative Sperm Membrane Associated Proteins by TX-114—Phase Partitioning
[0141]To isolate the hydrophobic, putative membrane associated sperm proteins, TX-114—phase partitioning was conducted. In particular, human sperm cells were solubilized in 1.7% TX-114 / TBS at 4° C. The solution was cetrifuged and the supernatant containing the solubilized proteins was recovered. TBS was added to adjust the TX-114 concentration to 1.% . Warming to 30° C. allowed the aggregation of micelles and separation of detergent phase from the aqueous phase. The two phases were separated by centrifugation.
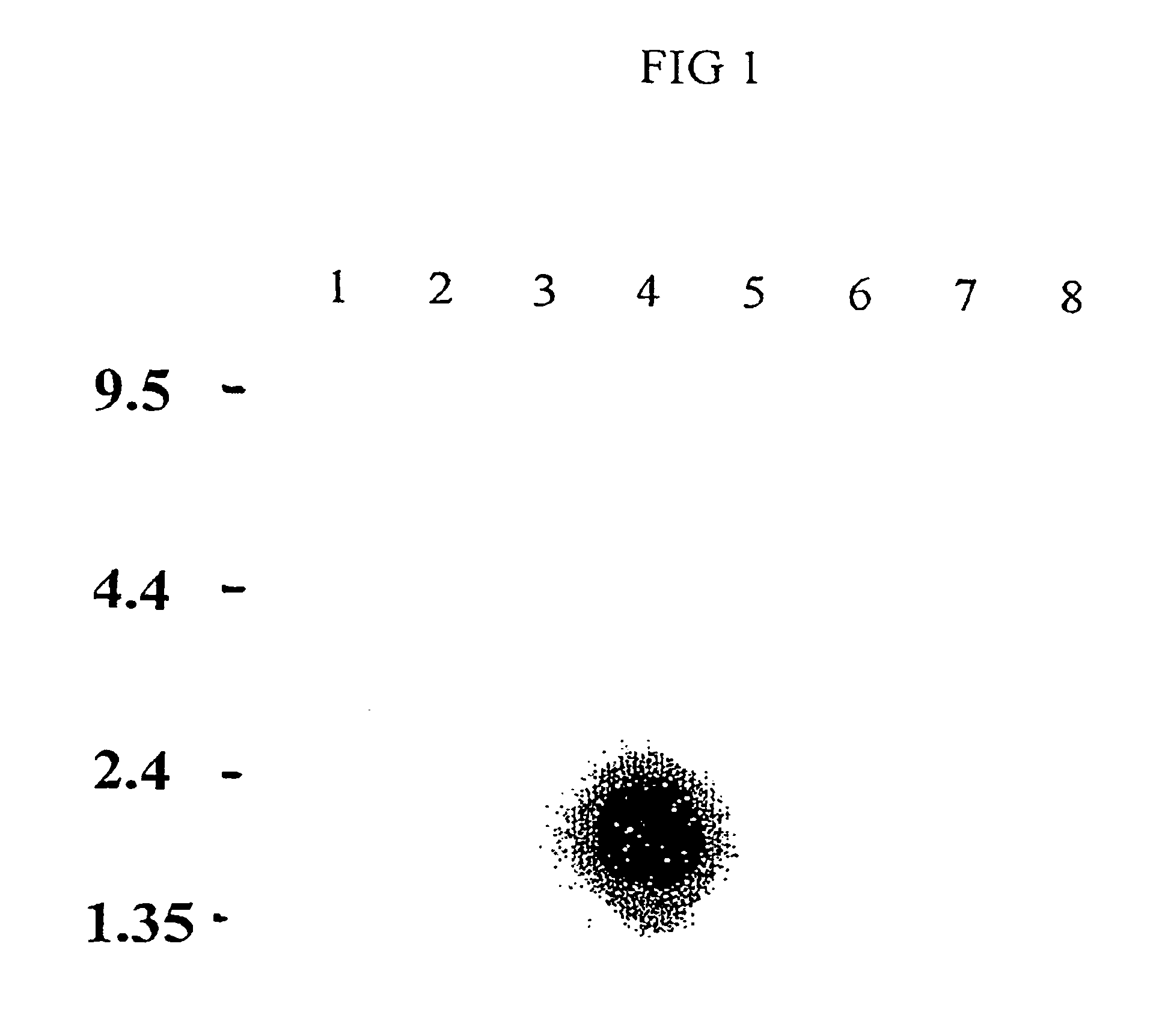
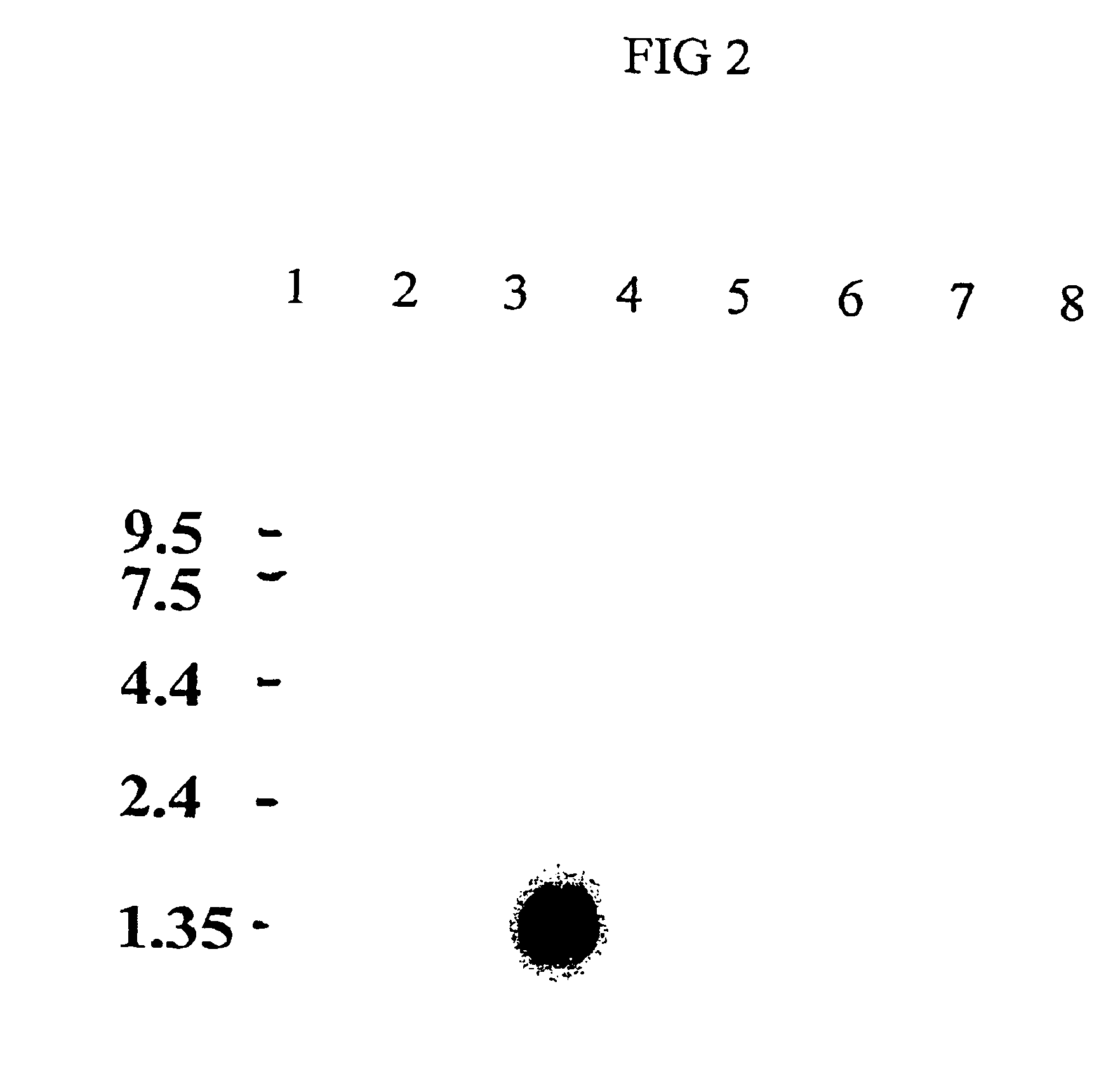
[0142]2-D electrophoresis was used to analyze the phase partitioned sperm proteins. The starting total extract, aqueous phase extract, and detergent phase extract were analyzed by 2-D gel electrophoresis. TX-114 phase partitioning allowed the selective partitioning of several hydrophobic proteins to the detergent phase. C58 is one of the 2-D protein spots enr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com