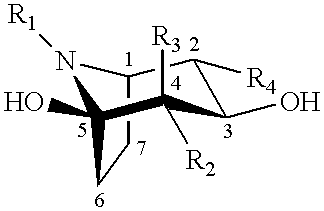
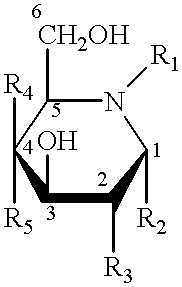
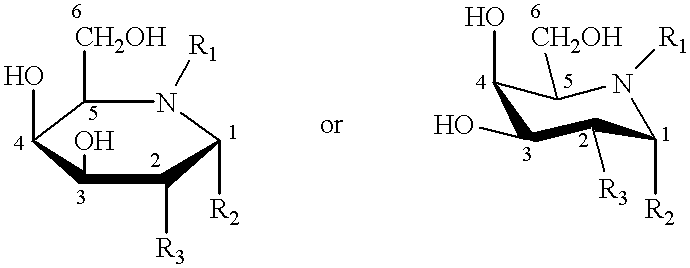Method of enhancing lysosomal alpha-Galactosidase A
a technology of lysosomal alpha-galactosidase and lysosomal alpha-galactosidase, which is applied in the direction of biocide, drug composition, cardiovascular disorder, etc., can solve the problem of excessive degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
DGJ, the strongest inhibitor in vitro and most effective intracellular enhancer, was chosen for more detailed characterization. DGJ was added to the TgM or TgN fibroblasts (FIG. 2A) and lymphoblasts derived from Fabry patients with genotypes of R301Q or Q279E mutations (FIG. 2B). The enzyme activity found in TgM fibroblasts increased 6-fold by co-cultivation with 20 .mu.M DGJ and reached 52% of normal. The DGJ also showed a similar effect on lymphoblasts in which the residual enzyme activity was enhanced by 8- and 7-fold in R301Q and Q279E, i. e., 48% and 45% of normal. The enzyme activity in Tg normal (TgN) fibroblasts and normal lymphoblasts also showed an increase by cultivation with DGJ.
example 3
The TgM fibroblasts and human lymphoblasts of normal and patient with a mutation on R301Q were cultured in the presence of DGJ at 20 .mu.M. In the cultures without DGJ, the .mu.-Gal A activity in TgM fibroblasts or mutant lymphoblasts was unchanged (FIG. 3). However, by including DGJ, the enzyme activity showed significantly increase in these cell cultures. The enzyme activity in mutant lymphoblasts reached to 64% of those found in normal lymphoblasts cultured without DGJ at the fifth day. The enzyme activity in normal lymphoblasts was also enhanced 30% after cultivation with DGJ.
example 4
DGJ concentration dependence of .alpha.-Gal A enhancement in transfected COS-1 cells, TgM fibroblasts and lymphoblasts with a phenotype of R301Q was examined.
As shown in FIG. 4, the enzyme activity increased with the increase in the concentration of DGJ in the range of 0.2-20 .mu.M in transfected COS-1 cells (FIG. 4A) and lymphoblasts (FIG. 4C), and between 0.2-200 .mu.M in TgM fibroblasts (FIG. 4B), respectively. A higher concentration of DGJ suppressed the enhancement effect.
DE-HNJ showed the same effect on the enhancement of .alpha.-Gal A in COS-1 cells transfected with a mutant cDNA of the enzyme (R301Q) at the higher concentrations (1-1000 .mu.M) compared with DGJ (FIG. 5). It is clear that DE-HNJ at 1 mM in culture medium did not inhibit intracellular enzyme activity of COS-1 cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com