Compositions and methods for platelet enriched fibrin constructs
a technology of fibrinogen and constructs, which is applied in the field of compositions and methods of platelet enriched fibrin contructs for delivery, can solve the problems of fibrinogen sealants, large handling difficulties of glues, and inability to harvest fibrinogen from small volumes of blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
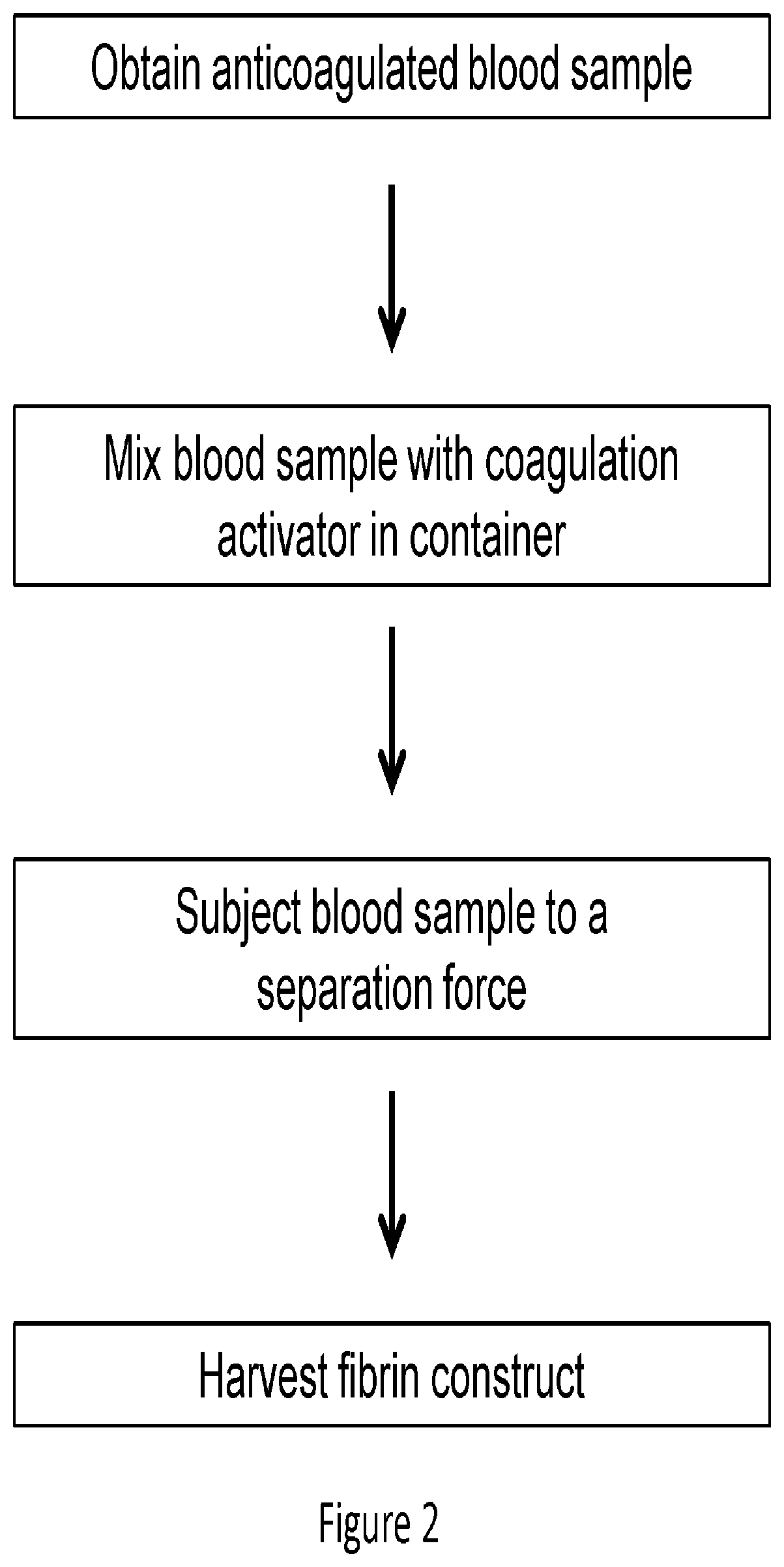
Preparatin of Platelet Enriched Fibrin Construct (PEFC)
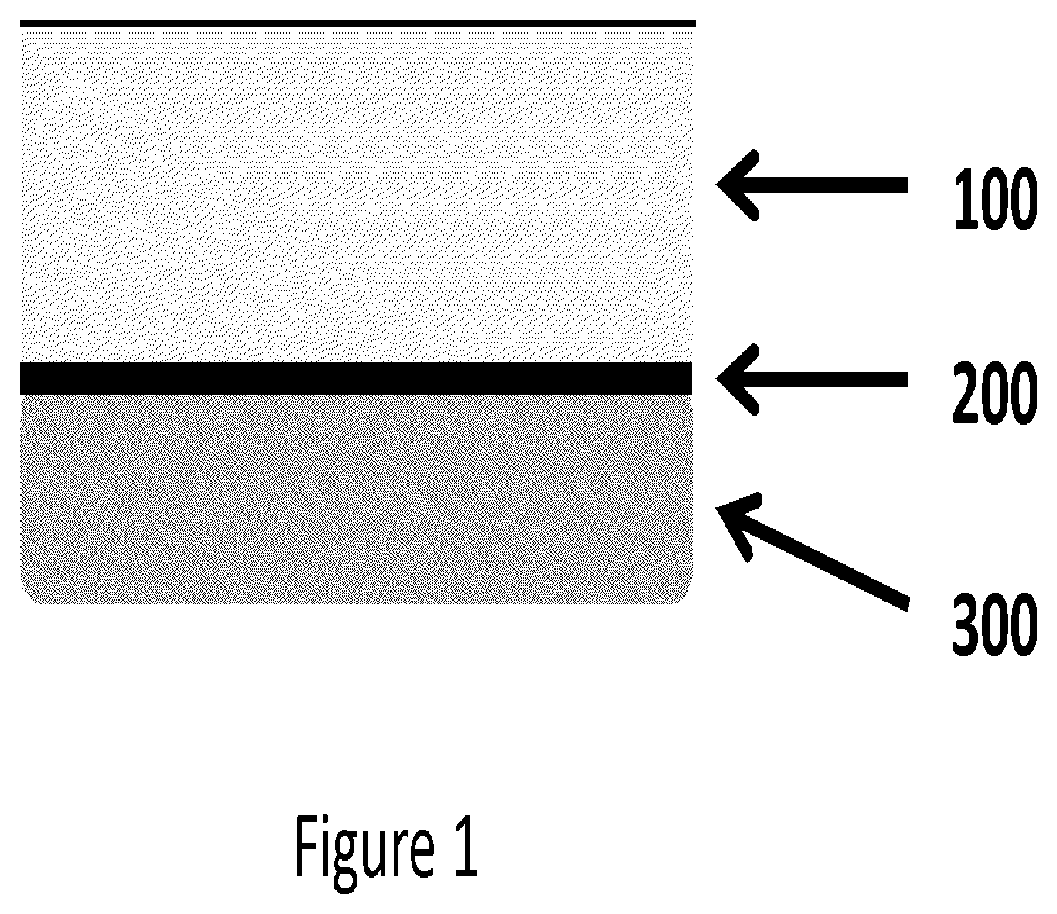
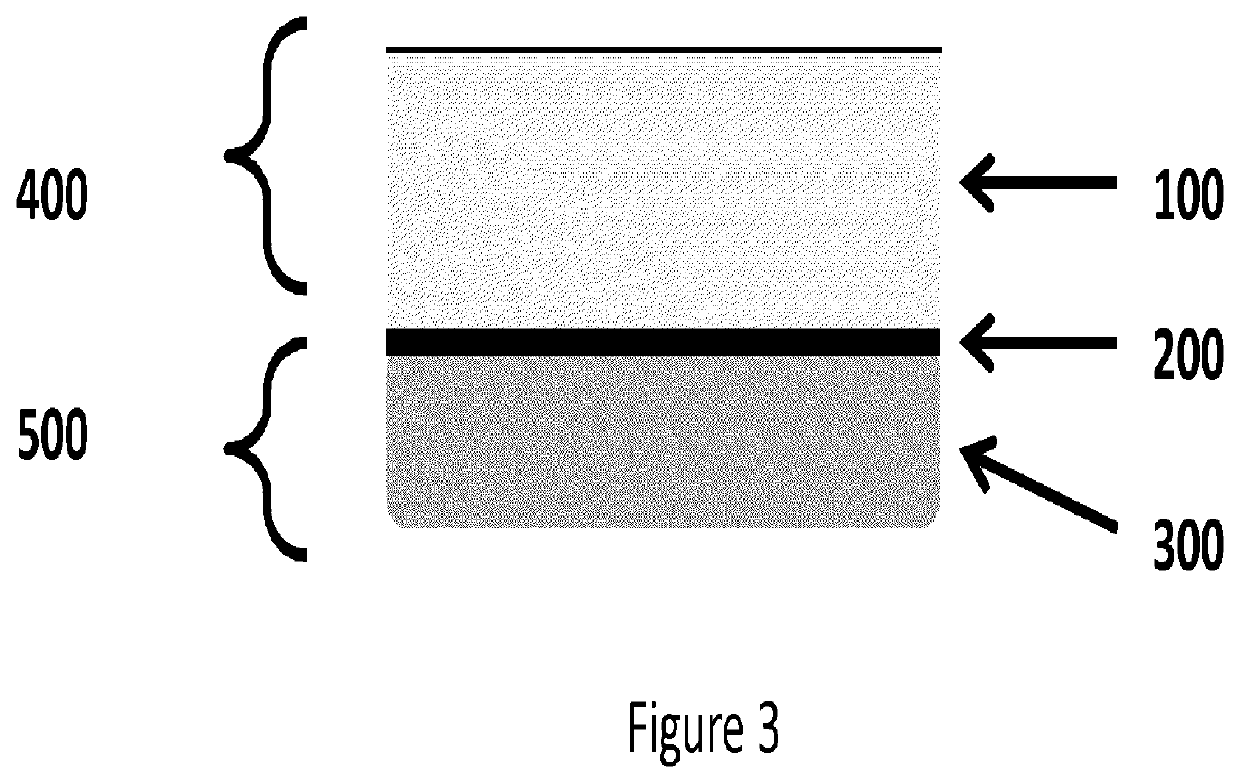
[0131]Soft Spin Preparations: Bovine blood samples of 6.8 ml were collected and exposed to 1.2 ml of anti-coagulant, ACD-A (15% by volume in final mixture). The blood samples were transferred to borosilicate glass tubes with 180 μl of 1M CaCl2 solution. The samples were inverted 10 times and centrifuged at 2000 xg for 10 mins. During centrifugation, the blood sample clotted and separated into distinct layers: a plasma layer (PLASMA), a fibrin plug layer (CLOT) and a blood cell layer (RBC). The images of FIG. 9 sequentially illustrate this procedure.
[0132]The blood cell layer can be physically removed by peeling the cap of blood cells, as shown in FIGS. 4A and 4B, from the fibrin layer, resulting in a construct comprising platelet / white blood cells, as shown in FIG. 4C. Also, FIG. 4D illustrates the blood cell cap removed from the fibrin layer. Most of the red blood cells can further be removed by blotting, scraping, etc.
[0133]Do...
example 2
Physical Properties of the Platelet Enriched Fibrin Construct
[0141]Physical property tests of the fibrin constructs were conducted with constructs prepared with bovine blood collected with ACD-A and shipped the previous day. A total of 18 samples were produced consecutively, with the maximum of 6 samples in centrifuge at a time for 15 minutes at 4500 RPM (2820 g) in capped borosilicate test tubes (with 180 μl of 1M CaCl2 solution). Upon the completion of the spin, the test tubes were immediately removed and placed vertically in holders. The clots, which were ˜14 mm in diameter, were extracted one at a time using forceps; excess proximal protein was removed, excess fluid was initially removed with gauze, and then the construct was trimmed to reduce thickness to ˜1 mm after a moderate amount of fluids was removed. The constructs were stored in DPBS solution until all samples were prepared and the DPBS fluids were removed from the constructs with light compression with gauze before mec...
example 3
Biochemical Properties of the Platelet Enriched Fibrin Construct
[0151]Histology: Human PEFC clots were generated according to the procedure of Example 1. Clots were gently blotted on kimwipes to remove excess fluid, cut into 3 sections and mounted in OCT compound on dry ice so that the cut edge created the plane for sectioning. Frozen cassettes were then sectioned in a cryostat ultra microtome at a thickness of approximately 5 microns. Tissues were immediately fixed in 100% methanol for 5 minutes at room temperature after mounting on glass slides. Slides were rinsed in phosphate buffered saline and then subjected to Wright-Giemsa staining following a standard whole blood smear staining protocol. Photomicrographs were obtained from the same field of view at increasing magnifications, as indicated in FIG. 15, using a Zeiss microscope outfitted with a digital color CCD camera.
[0152]FIG. 15 shows the stained sections of the PEFC at 50×, 100× and 200×. The upper non-stained portion is th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com