Hook fusion protein for regulating the cellular trafficking of a target protein
a technology of target protein and fusion protein, which is applied in the direction of animal/human proteins, peptides, antibody medical ingredients, etc., can solve the problems of affecting the transduction and/or generation of stable cell lines, affecting the production of lentiviruses, and affecting the transduction of proteins through ires
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
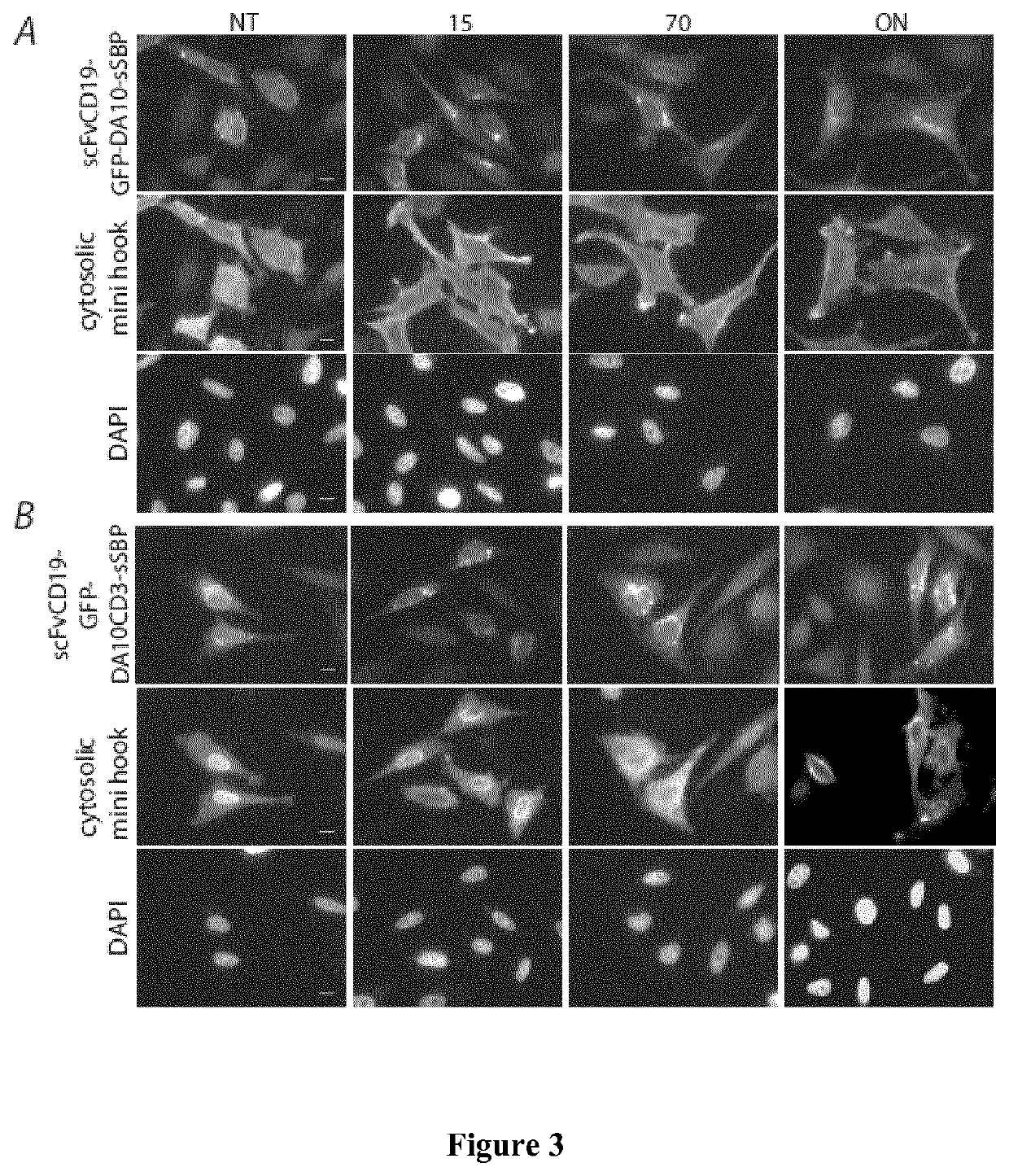
[0218]In the examples below, the term “Hook” refers to the hook fusion protein comprising the hook domain, and the term “Reporter” refers to the target membrane protein comprising the hook-binding domain.
[0219]Methods and Material:
[0220]Constructs
[0221]FIG. 1 shows a schematic representation of the Hook constructs by Boncompain et al (FIG. 1A), and of the new hooks according to the present invention (FIG. 1B, 1C). Those are inserted in the bicistronic vector using multicloning sites and the reporter using the typical cloning cassettes of the previously published RUSH vector. The soluble streptavidin containing an endocytosis signal and the ER retention signal KKXX were built by gene synthesis (gBlocks Gene Fragments—Integrated DNA Technologies or GeneArt / Thermo-Fisher). The soluble mini hook containing a endocytosis signal was synthetized by gene syntheses (gBlocks Gene Fragments—Integrated DNA Technologies) was generated by PCR amplification of the previously described luminal Ii-S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| soluble | aaaaa | aaaaa |
| nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com