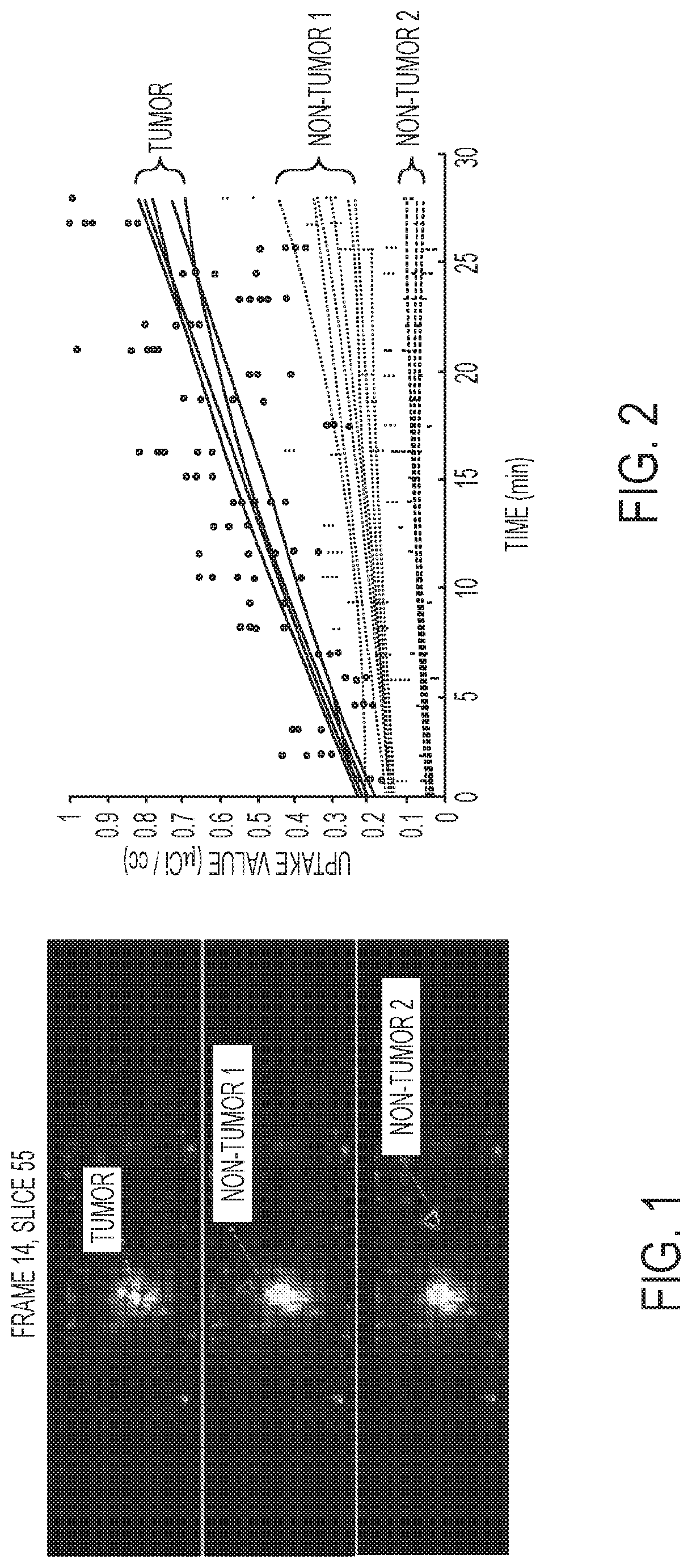
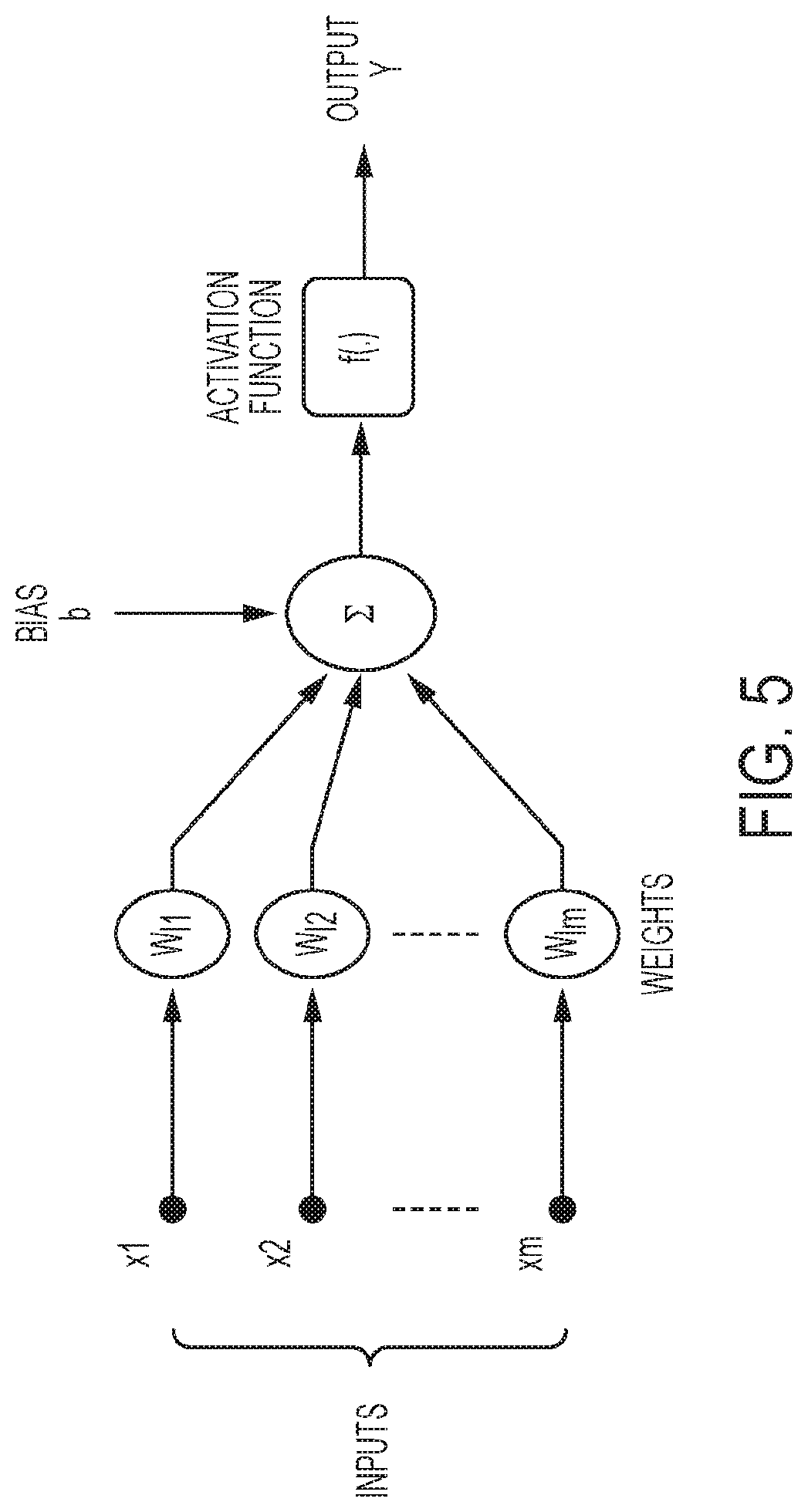
Automatic identification and segmentation of target regions in pet imaging using dynamic protocol and modeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0105]A continuous dynamic positron emission tomography (PET) assembly for imaging a target region of a subject. The assembly may comprise: a radioactive tracer isotope injector configured to administer a radioactive isotope into the subject; a scintillator crystal configured to absorb ionizing radiation from the subject and emit scintillator light, wherein said scintillator crystal undertakes the absorption substantially at the same time of the start of administering the radioactive isotope; a photo detector in communication with said scintillator crystal, wherein the photodetector is configured to detect the emitted scintillation light and provide electrical signals as output; a signal digitizing circuitry converting the output electrical signals into digital data; and a processor configured to receive the digital data and implement a model to convert the digital data into a three dimensional, tomographic image reconstruction.
example 2
[0106]The PET assembly of example 1, wherein said model comprises an artificial Neural Network (ANN).
example 3
[0107]The PET assembly of example 1 (as well as subject matter in whole or in part of example 2), wherein undertaking the absorption, by the scintillator crystal, substantially at same time of the start of administering the radioactive isotope includes at least one of the following ranges of timing:
[0108]about 1 minute to about 60 minutes after the start of administering;
[0109]about 5 minutes to about 30 minutes after the start of administering;
[0110]about 1 minute to about 25 minutes after the start of administering;
[0111]about 10 to about 30 minutes after the start of administering;
[0112]about 15 to about 20 minutes after the start of administering;
[0113]about 25 minutes after the start of administering;
[0114]about 30 to about 60 minutes after the start of administering; or
[0115]about 0 to 30 minutes after the start of administering;
[0116]about 25 to 30 minutes after the start of administering;
[0117]about 1 minute to about 10 minutes after the start of administering; or about 60 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com