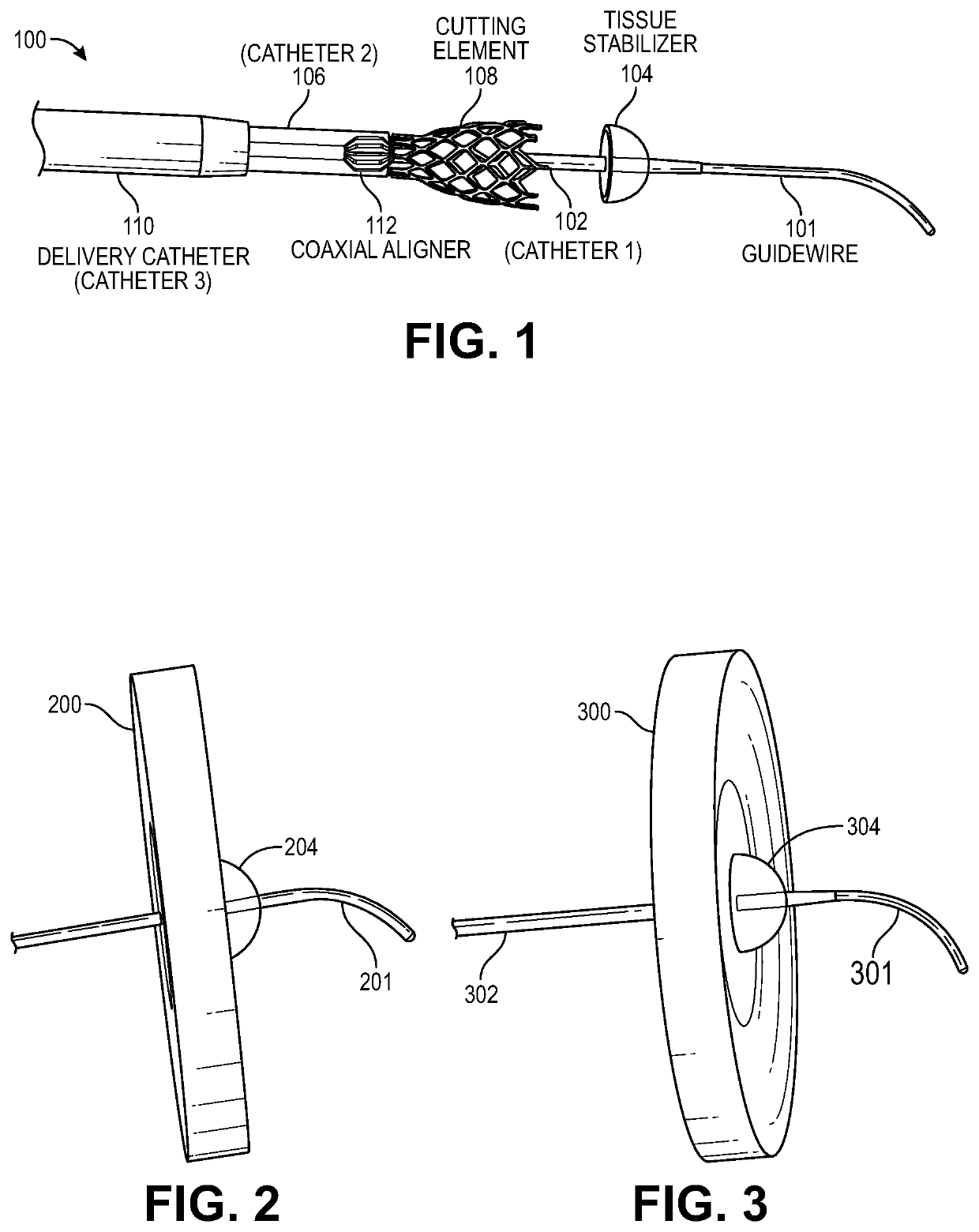
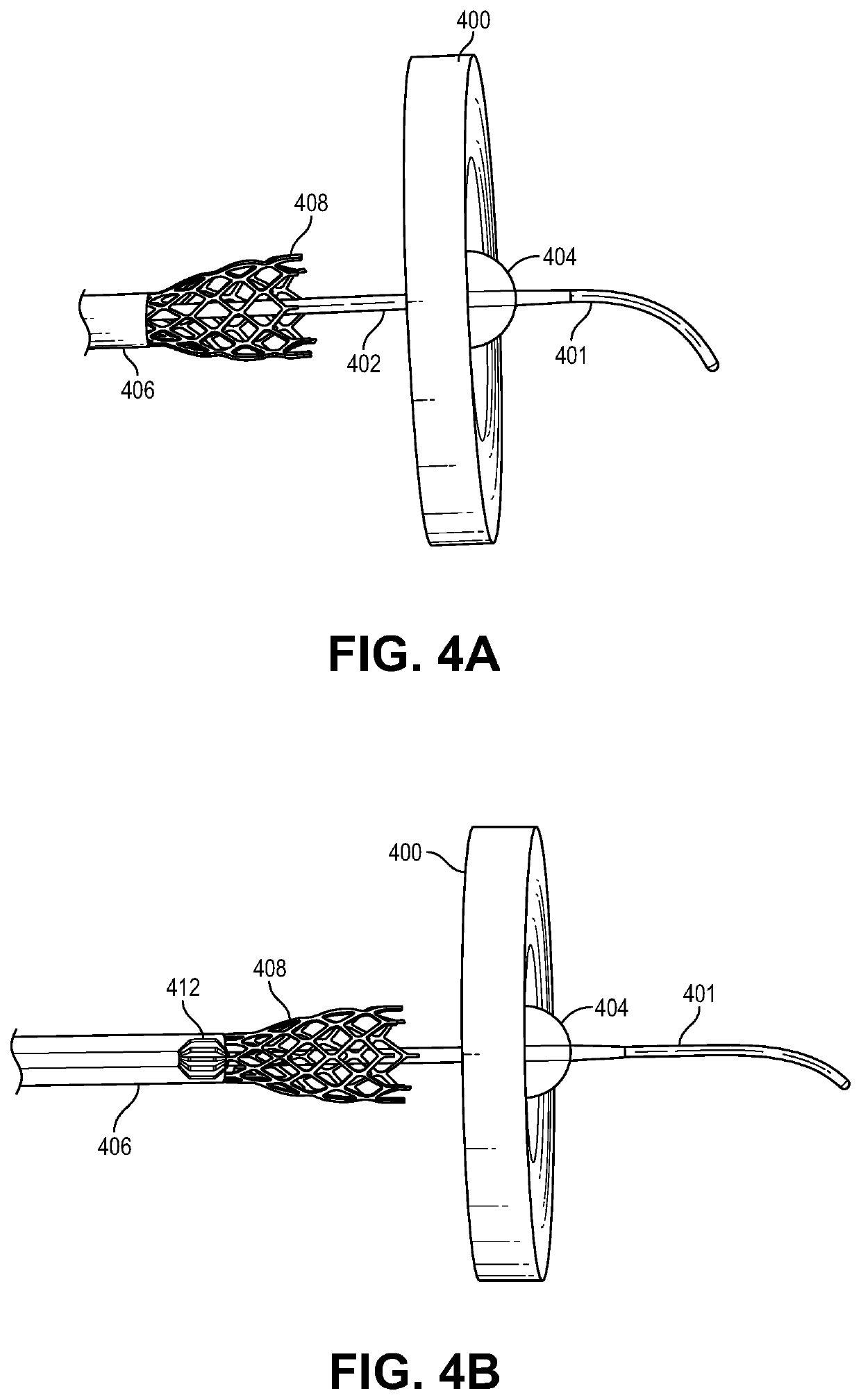
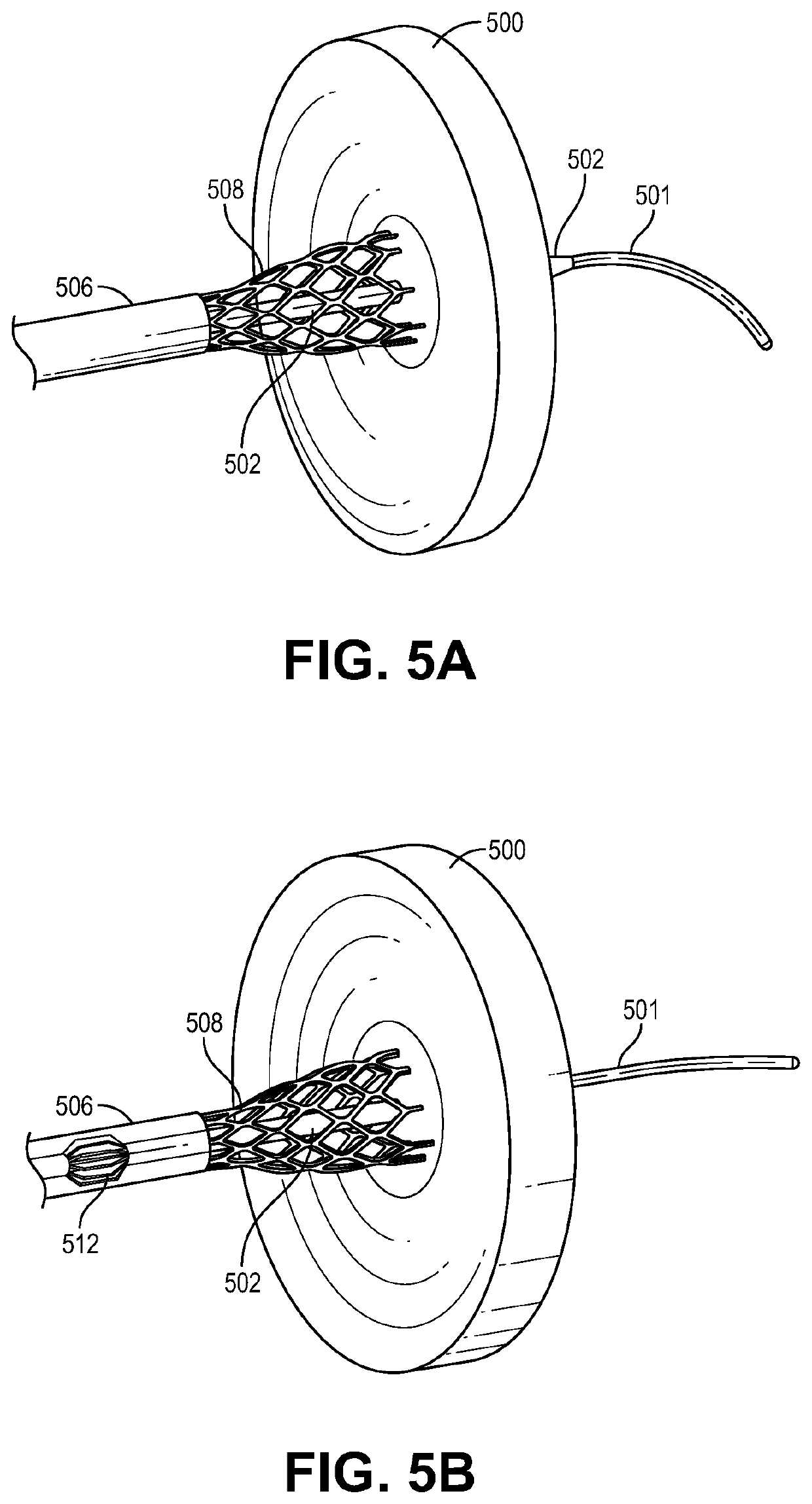
Transcatheter device for interatrial anastomosis
a transcatheter device and interatrial anastomosis technology, applied in the field of transcatheter devices for interatrial anastomosis, can solve the problems of significant associated healthcare costs and major epidemic of heart failure, and achieve the effects of increasing the size of the cutter, and slowing the natural healing process of the tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1400
[0189]In some embodiments, as shown in FIGS. 14A-14C, the tissue stabilizer embodiment 1400 is deployed from catheter 1, 1402, tracking along a guidewire (not shown) and again takes the form of a plurality of tines 1407 that pass through the septum as they were being deployed from the catheter 1, and expand in an umbrella fashion. The tines partially pierce through the septum 1420 (with or without barbs at the end of each tine) to prevent the septum from moving off of the tines. In either embodiment (with or without barbs on the tines), after the cutter 1408 is deployed from the delivery catheter 3 1410, and a portion of the interatrial septum is excised, the expanded tines permit resheathing within the delivery catheter, over the re-collapsed tines and excised tissue 1403, followed by removal of the device assembly from the body, as described previously. In some embodiments, the process of resheathing into the delivery catheter 1410, the tines 1407 bend backwards such that the tiss...
embodiment 1500
[0190]FIGS. 15A-15E are sequential representative illustrations of an exemplary embodiment of the tissue stabilizer taking the form of a loop supported by shape-set tines. Catheter 1, 1502 comprises a cap, 1523 having a central lumen that houses the collapsed tissue stabilizer. The tissue stabilizer catheter 1524, which is slidably engaged with the outside diameter of the catheter 1, 1502, is translated until its distal tip 1501, crosses the interatrial septum and allows for unsheathing of the catheter that comprises the tissue stabilizer in the left atrium. In some embodiments, as shown in FIGS. 15A-15E, the tissue stabilizer embodiment 1500 is collapsed and housed in catheter 1, 1502, that has a penetrating tip 1501, tracking along a guidewire (not shown) and takes the form of a loop supported by shape-set tines 1504, made with a shape memory metal or alloy. In some embodiments, catheter 1, 1502 comprises a cap 1522 and a shaft 1523. FIGS. 15A-15E are sequential representative ill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com