Gamma-hydroxybutyrate compositions and their use for the treatment of disorders
a technology of gamma-hydroxybutyrate and compositions, which is applied in the direction of anhydride/acid/halide active ingredients, medical preparations, nervous disorders, etc., can solve the problems of increasing the sodium intake of patients, and achieve the effect of reducing the level of growth hormone and increasing the intracranial pressur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Mixed Oxybate Solutions
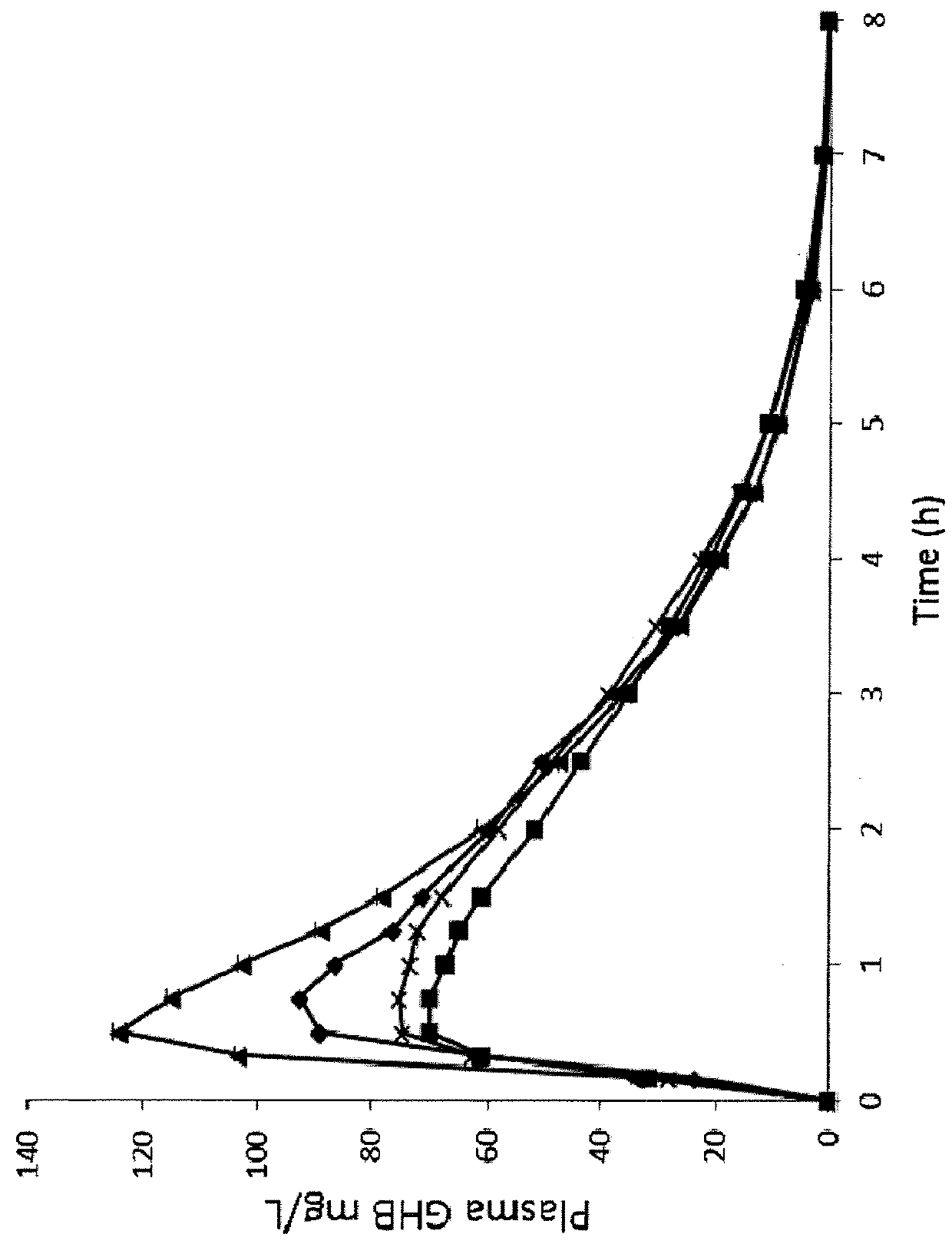
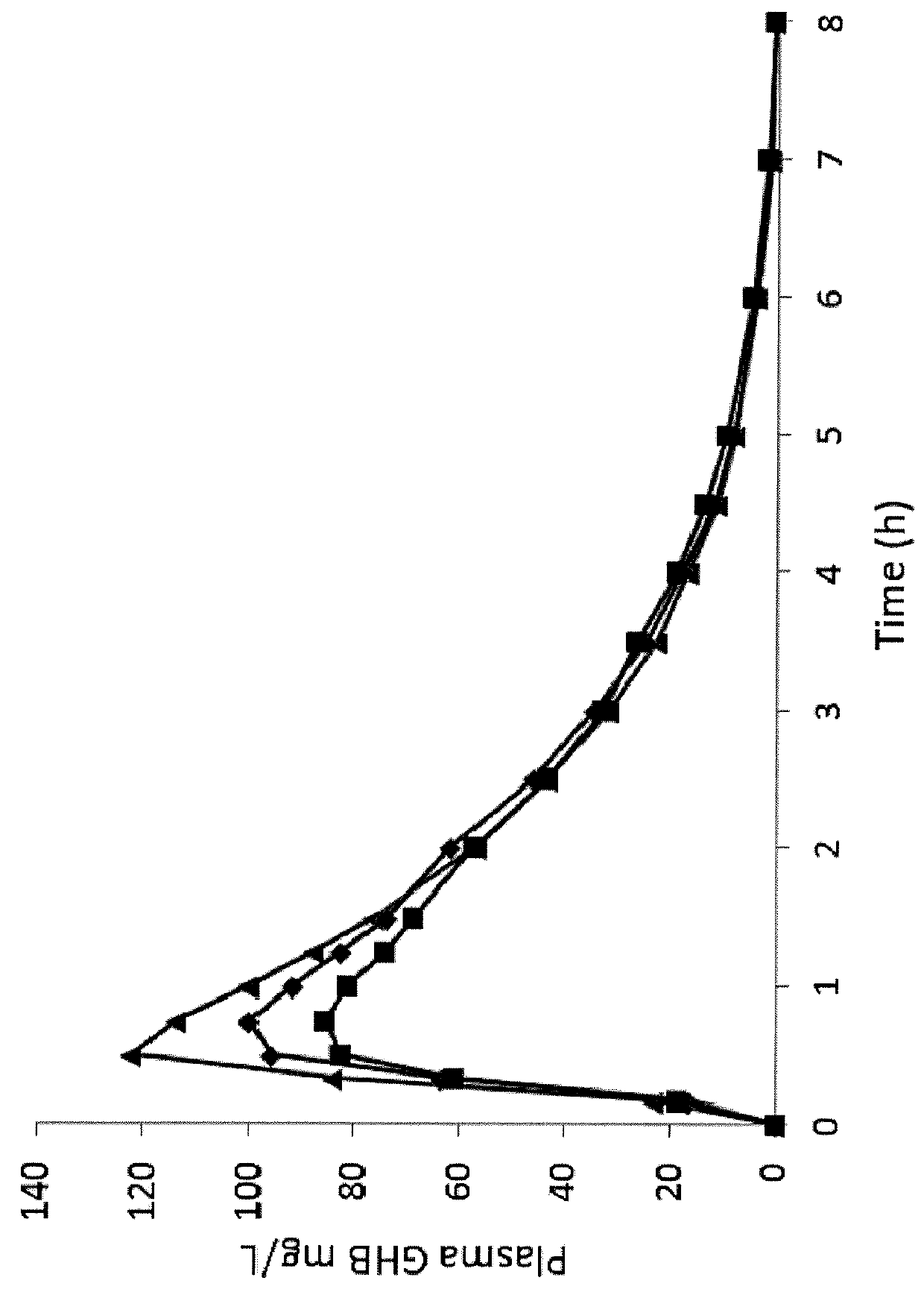
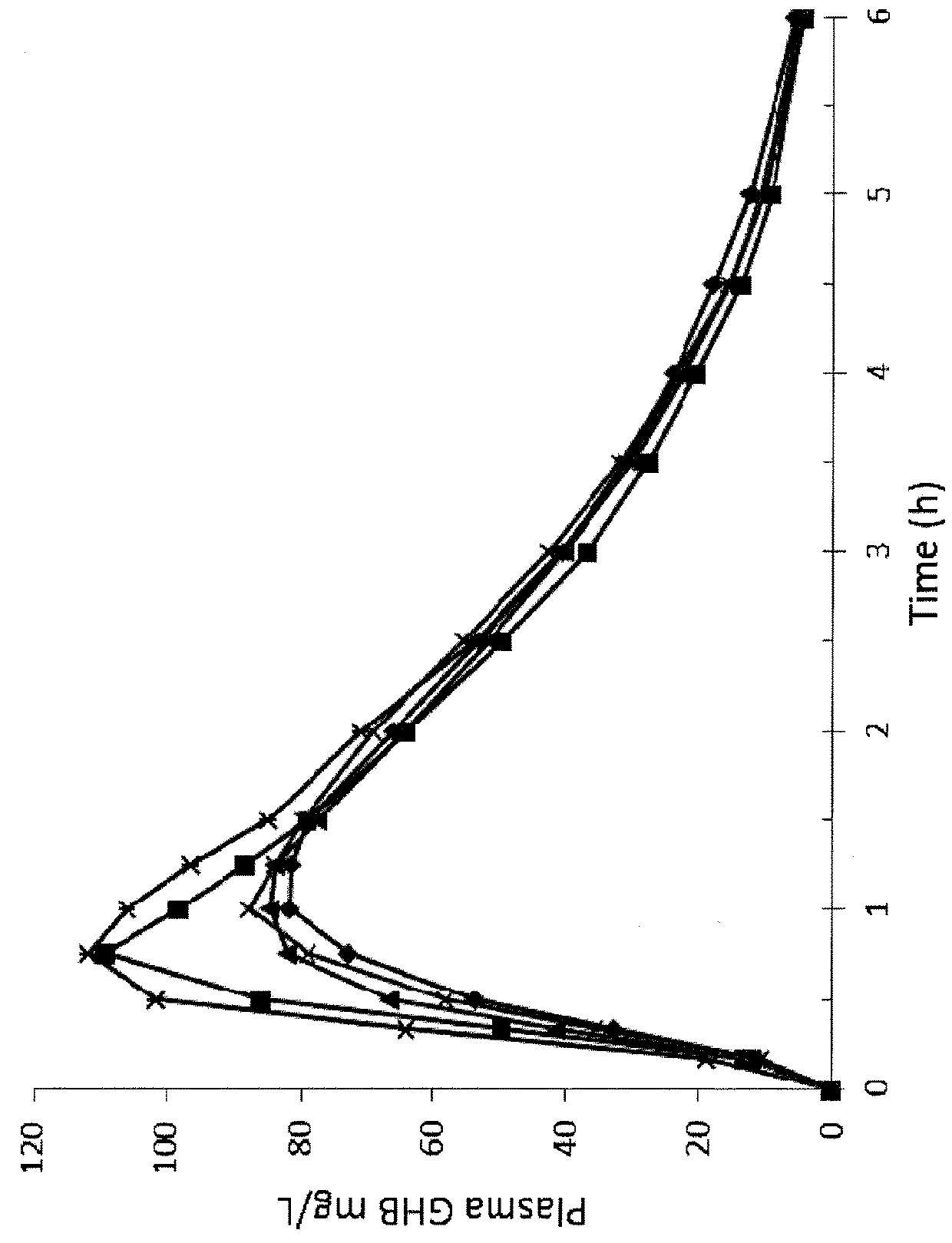
[0216]The following synthetic examples provide exemplary syntheses of mixture of oxybate salts. Alternate methods of synthesizing mixtures of oxybate salts, including methods of synthesizing additional salts of oxybate are described below; still other alternate synthetic methods will be apparent to those skilled in the art. See also U.S. Pat. Nos. 8,461,203; 8,591,922; 8,901,173; and 9,132,107; and U.S. Publication No. 2016 / 0058720; each of which is incorporated by reference in its entirety.
[0217]Mixed oxybate salt solutions can be made conveniently by at least two methods. When multiple different formulations are desired, one of skill in the art can mix solutions of individual salts having the same molar oxybate concentration to arrive at the desired cation blend. On the other hand, for commercial implementation or single-batch manufacturing one can perform a one-pot reaction with GBL and the two or more bases in the desired cationic proportions. Both meth...
example 1.1
ng Mixed Salts Solutions
[0219]Four individual oxybate salt solutions at equal oxybate strength (409 mg / mL) were made as follows:
[0220]Magnesium oxybate (Mg.(GHB)2) solution was made by combining 124.6 g water and 20.36 g magnesium hydroxide in a magnetically-stirred 250 mL square glass bottle. 58.04 g of GBL was then added to the base suspension and then heated up to 80° C. with stirring. After 4 hours, a pH verification indicated completion of reaction (pH 8.5). Water was added to compensate for evaporation. The reaction mixture was then centrifuged, and supernatant filtered through 0.45p PVDF Stericup under vacuum. The pH of filtrate was 8.1. Yield: 177.4 g solution. Assay (HPLC-UV): 100.1%
[0221]Potassium oxybate (K.GHB) solution was made by adding 60.10 g potassium hydroxide to 144.01 g water in a magnetically-stirred 250 mL square glass bottle. After complete dissolution, 78.52 g GBL was weighed into a separate glass beaker. Approximately half the GBL was added initially with in...
example 1.2
-Pot Reaction Method to Achieve Various Mixtures
[0225]To achieve any combination of oxybate salts, the stoichiometry calculations are adjusted to reflect (a) the strength of individual bases and (b) the use of an excess for the weakest base (calcium or magnesium). The strength of bases used in the Example above were 99.7% (NaOH), 86.0% (KOH), 99.0% (Ca(OH)2), and 98.5% (Mg(OH)2). A 1% excess is applied as the weakest divalent base present (calcium or magnesium, in that order of precedence). A larger or smaller excess may be warranted, depending on the level of confidence in the assay values or repeatability of dispensing to the process. A larger excess will increase confidence in completing the reaction, but incur more filtration load. A smaller excess threatens to inadequately complete the reaction, resulting in higher than desired GBL levels.
[0226]To make 150 mL batches roughly equivalent in composition to those of Example 1.1, the stoichiometry is as shown in Table 3 below.
TABLE ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com