Radiopharmaceutical solutions with advantageous properties
a radiopharmaceutical and solution technology, applied in the field of radiopharmaceutical solutions, can solve the problems of dna destruction by a high degree of irreparable double strand break, the use of aqueous solution was finally abandoned, and the use of dna was finally abandoned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
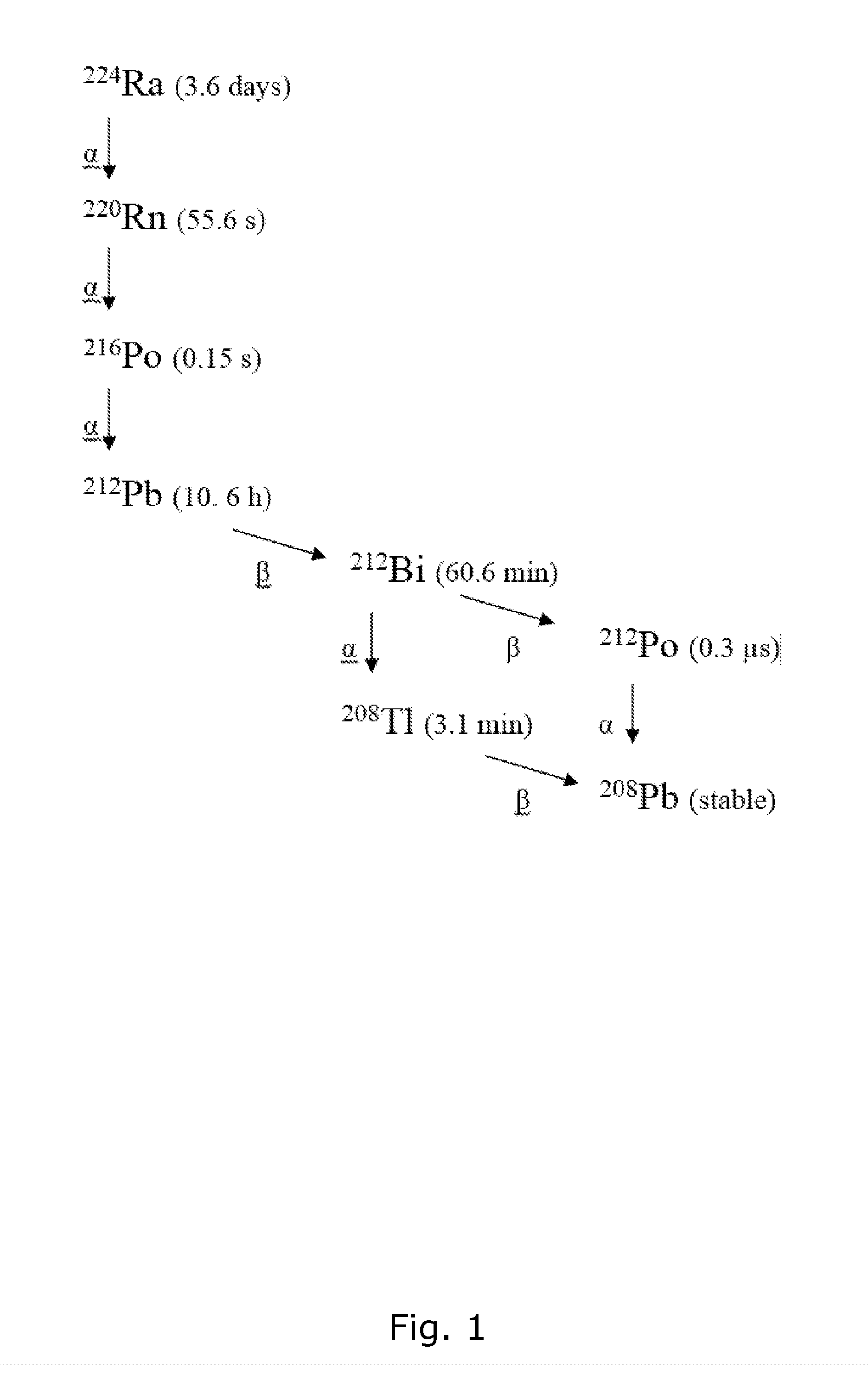
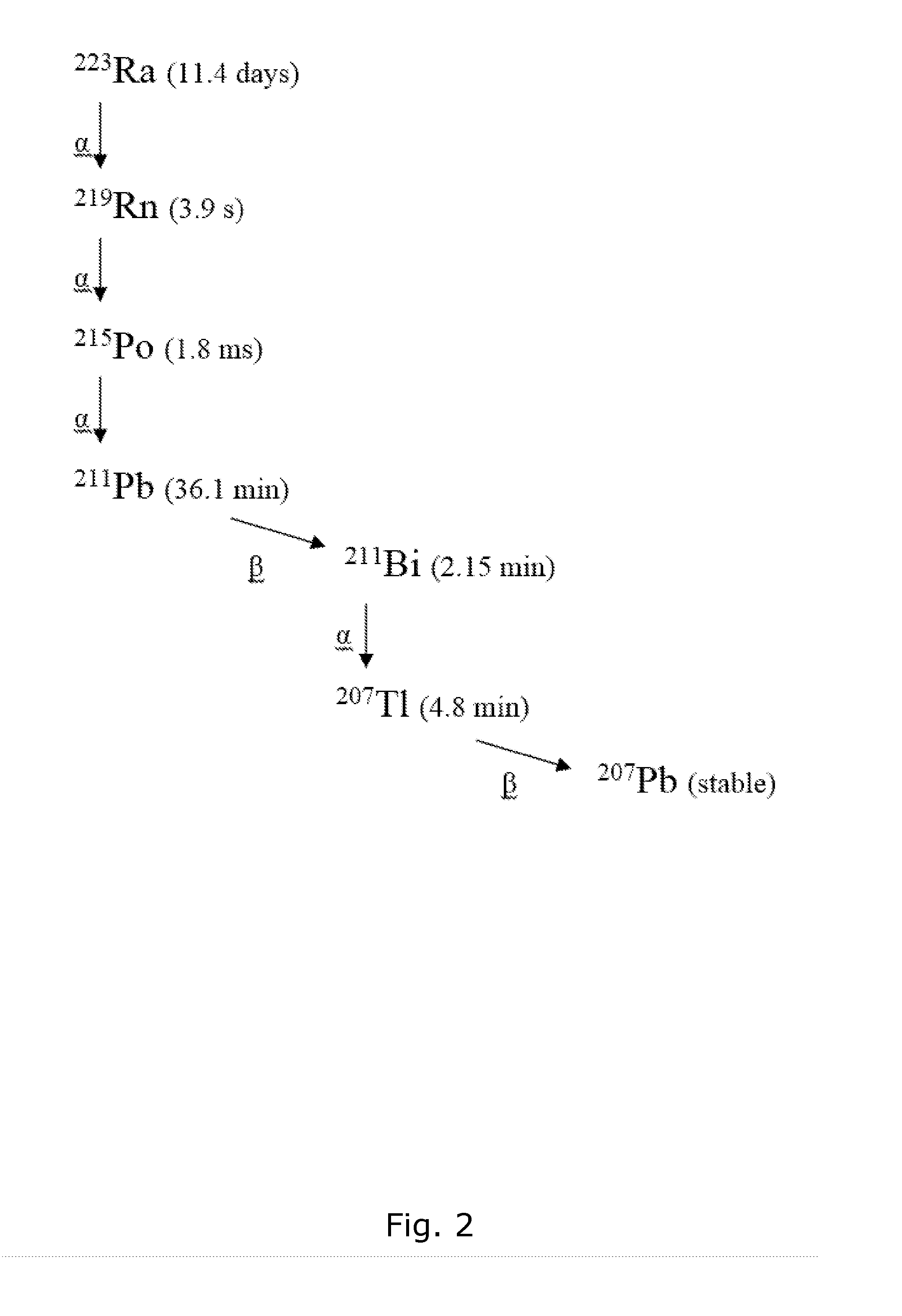
Calculation of the 212Pb Daughter Nuclide Level from 224Ra Decay at Various Time Points
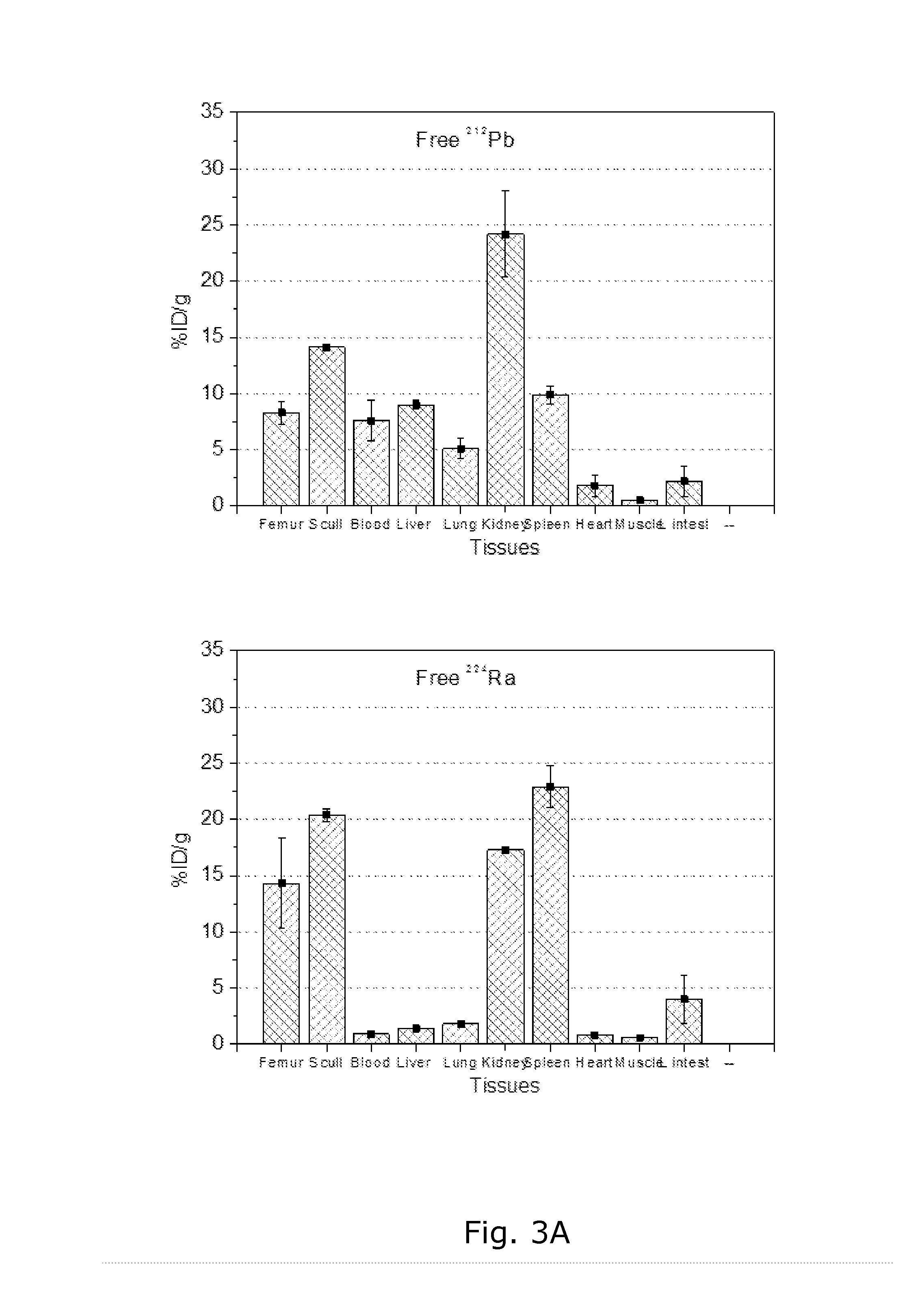
[0136]Background. The 212Pb produced after preparation of a pure 224Ra radiopharmaceutical may be a problem since it has different and undesirable properties compared with the mother nuclide. E.g., it is known that radium can target bone and bone metastases, but the lead progeny has undesired accumulation in hematopoietic cells and tissues and in the kidneys.
[0137]Method: The ingrowth of 212Pb from a pure 224Ra source were calculated using a universal activity calculator.
[0138]Results: Table 2 shows the amount of 212Pb at various time points after the production of a pure 224Ra pharmaceutical solution and storage in a gas tight container.
[0139]The data shows that significant amount of daughter nuclide is present within a relatively short time frame, complicating a potential centralized production and supply of 224Ra based radiopharmaceuticals. It is noteworthy though that the ratio of 212Pb to 224...
example 2
Preparation of Radionuclides and Counting of Radioactive Samples
[0140]In the following, all work with the concentrated radioactive preparations including evaporation of solvent etc was performed in a glove-box. A source of 228Th in 1 M HNO3 was acquired from a commercial supplier. Ac-resin was obtained from Eichrom Technologies LLC (Lisle, Ill., USA) in the form of a pre-packed cartridge.
[0141]To use smaller volume of solvent, about thirty percent of the materials in a cartridge (Cartridge 1) was extracted and repacked in a smaller column (Cartridge 2) made by a 1 ml filtration column (Isolute SPE, Biotage AB, Uppsala, Sweden). A slurry representing 20% of the original cartridge content was used for immobilizing of 228Th in 500 mikroliter 1 M HNO3 which was added 500 microliter of 1 M HCl and incubated by shaking the vial (4 ml vial, E-C sample, Wheaton, Millville, N.J., USA) for at least 4 hours. Cartridge 2 was added a small amount (about 0.1 ml) of the Ac-resin. Thereafter, the s...
example 3
Determining Net Count Rate for 212Pb in a 212Pb / 224Ra Mixture Before Radioactive Equilibrium has been Reached
[0145]After more than 3 days, i.e., “equilibrium” a sample will for practical purposes have 1.1 times 212Pb vs 224Ra.
[0146]Regardless of whether 212Pb is higher or lower than equilibrium it can be assumed that this is reached after 3 days since surplus 212Pb is reduced by 99% and the ingrowth of 212Pb from 224Ra is practically complete vs. “equilibrium”.
[0147]Using the Cobra II Autogamma counter with a counting window setting from 70-80 KeV gives mainly the 212Pb with very little contribution from other radionuclides in the 224Ra series. Radium-224 must be indirectly counted when the initial 212Pb has vanished and equilibrium between 224Ra and 212Pb has been reached (after approximately 3 days). This indirect counting requires the sample to be stored in a relatively gas tight containers as otherwise the 220Rn may escape preventing the radionuclide equilibrium of 1.1 between 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com