Aluminum chelate compound and room temperature-curable resin composition containing same
a technology of aluminum chelate and resin composition, which is applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, other chemical processes, etc., can solve the problems of insufficient fast curability, low activity insufficient heat resistance of aluminum chelate compound, etc., to achieve superior heat resistance, water resistance and moisture resistance, superior catalyst activity, and preservation stability. the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working example
[0105]Next, working and comparative examples are shown to describe the present invention in detail. However, the present invention is not limited to the following working examples.
working example 1
[0106]Aluminum triethoxide of 0.81 g (5.0 mmol) and toluene of 2.0 ml were placed in a 50 ml eggplant-shaped flask, followed by delivering thereinto 1.58 g (10.0 mmol) of 4,4-dimethyl-3-oxopentanoic acid methyl and then 0.50 g (5.0 mmol) of 2,4-pentanedione by drops while performing stirring. After performing stirring under a room temperature for 24 hours, the ethanol generated was then distilled to obtain 2.20 g (yield 100%) of a yellow viscous fluid having an average structure of that of a monoacetylacetonate aluminum bis (methylpivaloylacetoacetate) chelate.
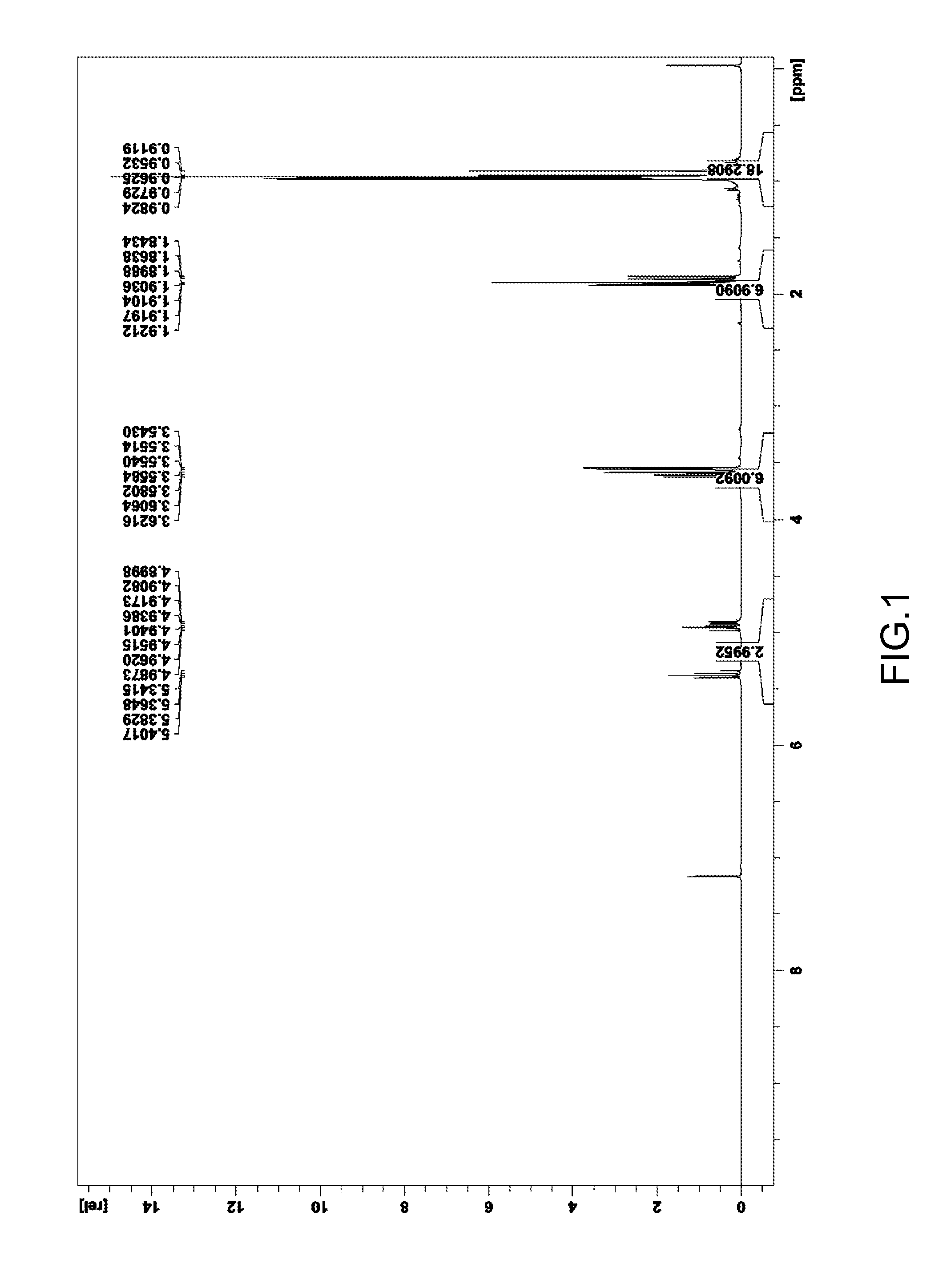
[0107]A measurement through 1H-NMR spectrum was performed (FIG. 1) to confirm the average structure of the product generated.
[0108]1H-NMR spectrum:
[0109]δ 0.91 to 0.98 ppm (ratio of H: 6, —C(O)—C(CH3)3)
[0110]1.84 to 1.92 ppm (ratio of H: 6, —C(O)—CH3)
[0111]4.11 to 4.18 ppm (ratio of H: 6, —OCH3)
[0112]4.90 to 5.40 ppm (ratio of H: 3, —C(O)CHC(O)—)
[0113]According to the results of the measurement through 1H-NMR spectrum, it is r...
working example 2
[0114]Aluminum triethoxide of 0.81 g (5.0 mmol) and toluene of 2.0 ml were placed in a 50 ml eggplant-shaped flask, followed by delivering thereinto 1.84 g (10.0 mmol) of 4,4,4-ethyl trifluoroacetoacetate and then 0.50 g (5.0 mmol) of 2,4-pentanedione by drops while performing stirring. After performing stirring under a room temperature for 24 hours, the ethanol generated was then distilled to obtain 2.46 g (yield 100%) of a yellow viscous fluid having an average structure of that of a monoacetylacetonate aluminum bis (ethyl-4,4,4-trifluoroacetoacetate) chelate.
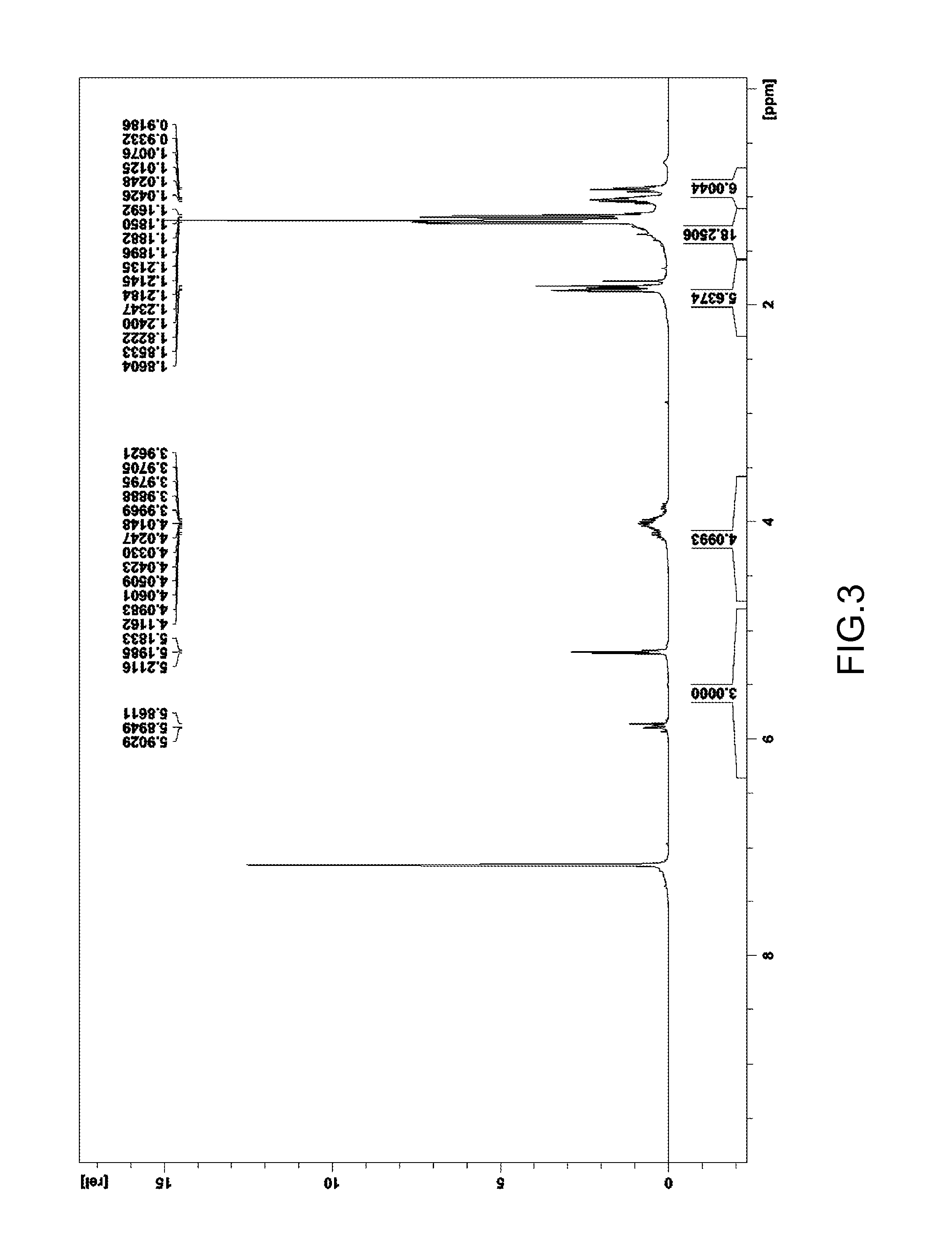
[0115]A measurement through 1H-NMR spectrum was performed (FIG. 2) to confirm the average structure of the product generated.
[0116]1H-NMR spectrum:
[0117]δ 1.17 to 1.21 ppm (ratio of H: 6, —OCH2CH3)
[0118]1.89 to 1.93 ppm (ratio of H: 6, —C(O)—CH3)
[0119]4.11 to 4.18 ppm (ratio of H: 4, —OCH2CH3)
[0120]5.29 to 5.43 ppm (ratio of H: 3, —C(O)CHC(O)—)
[0121]According to the results of the measurement through 1H-NMR spectrum, it is re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com