Selective UV crosslinking of peptides and functional moieties to immunoglobulins
a functional moiete and immunoglobulin technology, applied in the field of selective uv crosslinking of peptides and functional moieties to immunoglobulins, can solve the problems of reducing sensitivity and reproducibility, heterogeneous immunoglobulin population, and non-site-specific conjugation methods that have negative effects on the outcome of immunoglobulin-based detection assays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Nucleotide Binding Site Conjugation
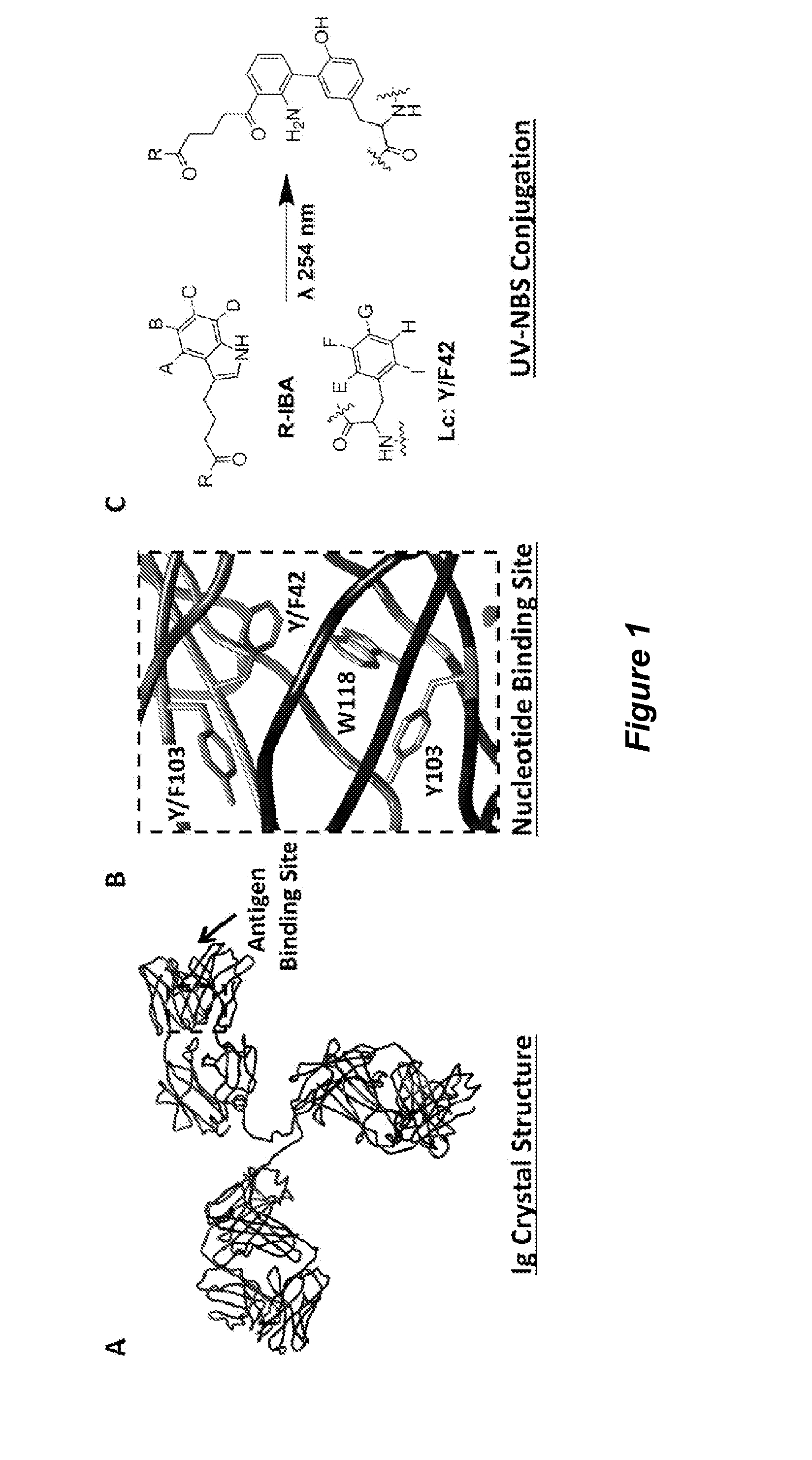
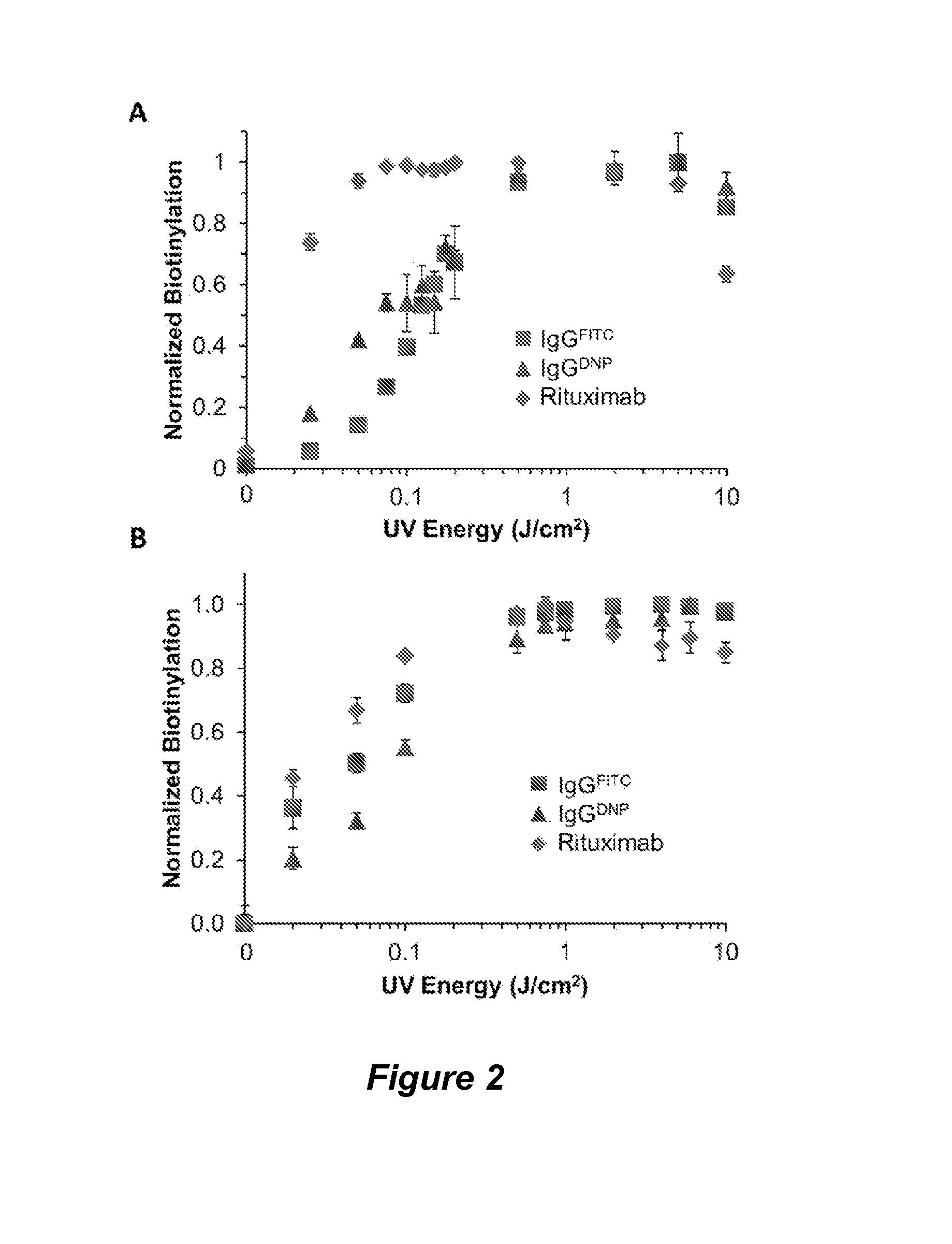
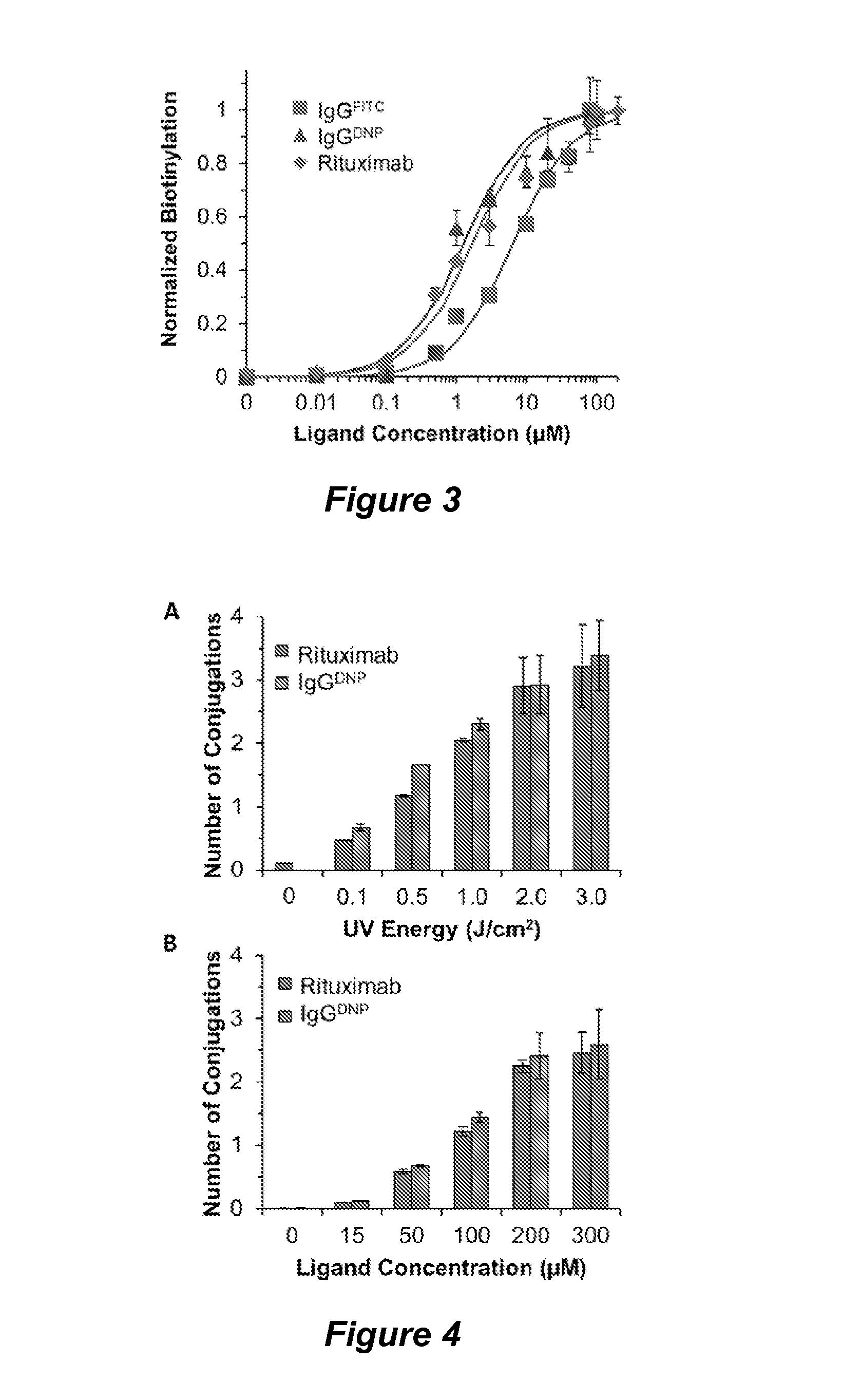
[0184]The nucleotide binding site (NBS) provides a useful site for selective conjugation of immunoglobulins to small ligands that contain aromatic rings to selectively bind this site. To identify such small molecules with a high binding affinity and selectivity for the NBS, we performed an in silico screening by docking various small molecules from the ZINC database at the NBS. The top scoring molecules were then experimentally investigated for their binding affinity to the NBS with indole-3-butyric acid (IBA) emerging as the highest affinity binding nucleotide analogue, with Kd values ranging between 1 and 8 μM depending on the immunoglobulin. Consequently, the IBA conjugated versions of functional ligands (IBA-ligand) such as affinity tags, fluorescent molecules, peptides, and chemotherapeutics can be photocrosslinked to immunoglobulins site-specifically at the NBS.
[0185]In this example we have particularly demonstrated the site-specific function...
example 2
UV-NBS Photocrosslinking of Reactive Thiol Moieties
[0246]The UV-NBS photocrosslinking technique requires exposure to UV energy that some functional ligands may not be stable to. In this study, we demonstrate the utility of the UV-NBS immunoglobulin functionalization strategy for conjugation of reactive thiol ligands to immunoglobulins at their NBS. By synthesizing an IBA conjugated version of cysteine (IBA-Thiol) a reactive thiol group can be site-specifically photocrosslinked to immunoglobulins at the NBS (FIG. 32). This thiol group can then be used as an orthogonally reactive site to conjugate UV sensitive functional ligands that possess either a thiol reactive group resulting in disulfide bond formation or subsequent reaction with a maleimide functionalized ligand (FIG. 28). The results detailed here provide a universal technique for the site-specific conjugation of UV sensitive functional ligands to immunoglobulins at the NBS, while preserving immunoglobulin activity.
[0247]Mater...
example 3
UV-NBS Photocrosslinking of Biotin
[0267]Here, we describe an alternate photochemistry based NBS-specific immunoglobulin immobilization method that utilizes biotin for oriented immobilization to streptavidin-functionalized surfaces (UV-NBSBiotin, FIG. 40). We predicted that site-specifically conjugating a biotin molecule to the immunoglobulin NBS prior to immobilization would allow for nearly 100% immunoglobulin functionalization to overcome the poor immobilization efficiency of the previously reported UV-NBS method while still maintaining maximum immunoglobulin activity.
[0268]1. Materials
[0269]IBA, Biotin N-hydroxysuccinimide ester (NHS-Biotin), N,N-Diisopropylethylamine (DIEA), were purchased from Sigma-Aldrich (St. Louis, Mo.). Streptavidin-HRP and HRP-conjugated IgG Fcγ specific goat anti-mouse were purchased from Jackson ImmunoResearch (West Grove, Pa.). Heat shock isolated bovine serum albumin (BSA), Amicon Ultra centrifugal filters (0.5 mL, 10K) and Coomassie R-250 were purcha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com