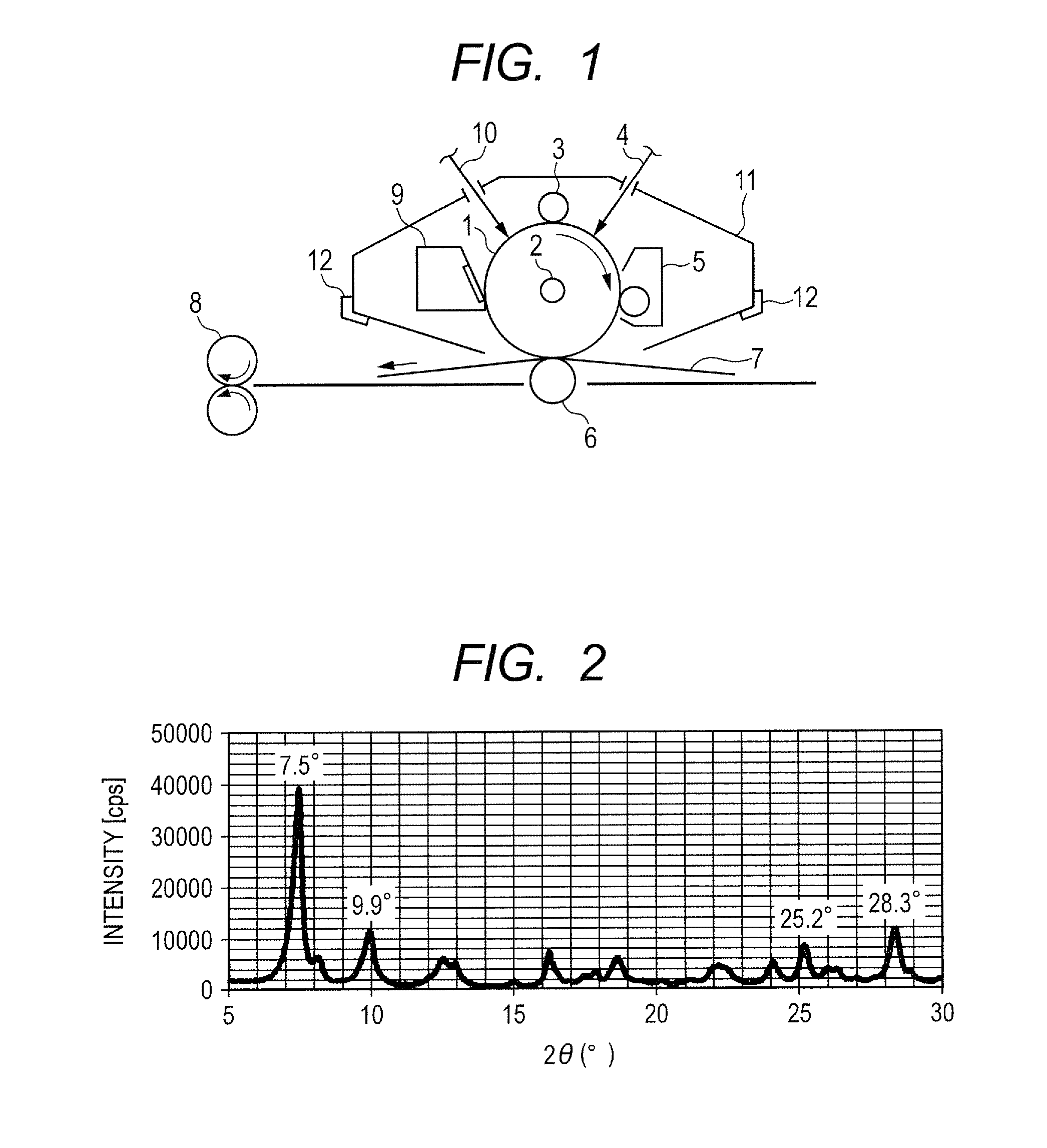
Electrophotographic photosensitive member, manufacturing method of electrophotographic photosensitive member, process cartridge and electrophotographic apparatus, and phthalocyanine crystal and manufacturing method of phthalocyanine crystal
a manufacturing method and photosensitive technology, applied in the field of electrophotographic photosensitive members, can solve the problems of potential variation and difficulty in conversion to a desired crystalline form in some cases, and achieve the effect of reducing image defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-1
[0076]As described below, hydroxygallium phthalocyanine was produced by the same as in Synthesis Example 1 and Example 1-1 described in Japanese Patent Application Laid-Open No. 2011-94101. Under nitrogen flow atmosphere, 5.46 parts of phthalonitrile and 45 parts of α-chloronaphthalene were fed into a reaction tank, then heated up to a temperature of 30° C., and maintained at the temperature. Subsequently, 3.75 parts of gallium trichloride was fed thereto at the temperature (30° C.). At the feeding time, the mixture liquid had a water content of 150 ppm. The temperature was then increased to 200° C. Under the nitrogen flow atmosphere, a reaction was caused at a temperature of 200° C. for 4.5 hours, which was then cooled to a temperature of 150° C. for filtering a product. The produced residue was dispersed and cleaned with N,N-dimethylformamide at a temperature of 140° C. for 2 hours, and then filtrated. The produced residue was cleaned with methanol and dried to produce 4.65 parts ...
example 1-2
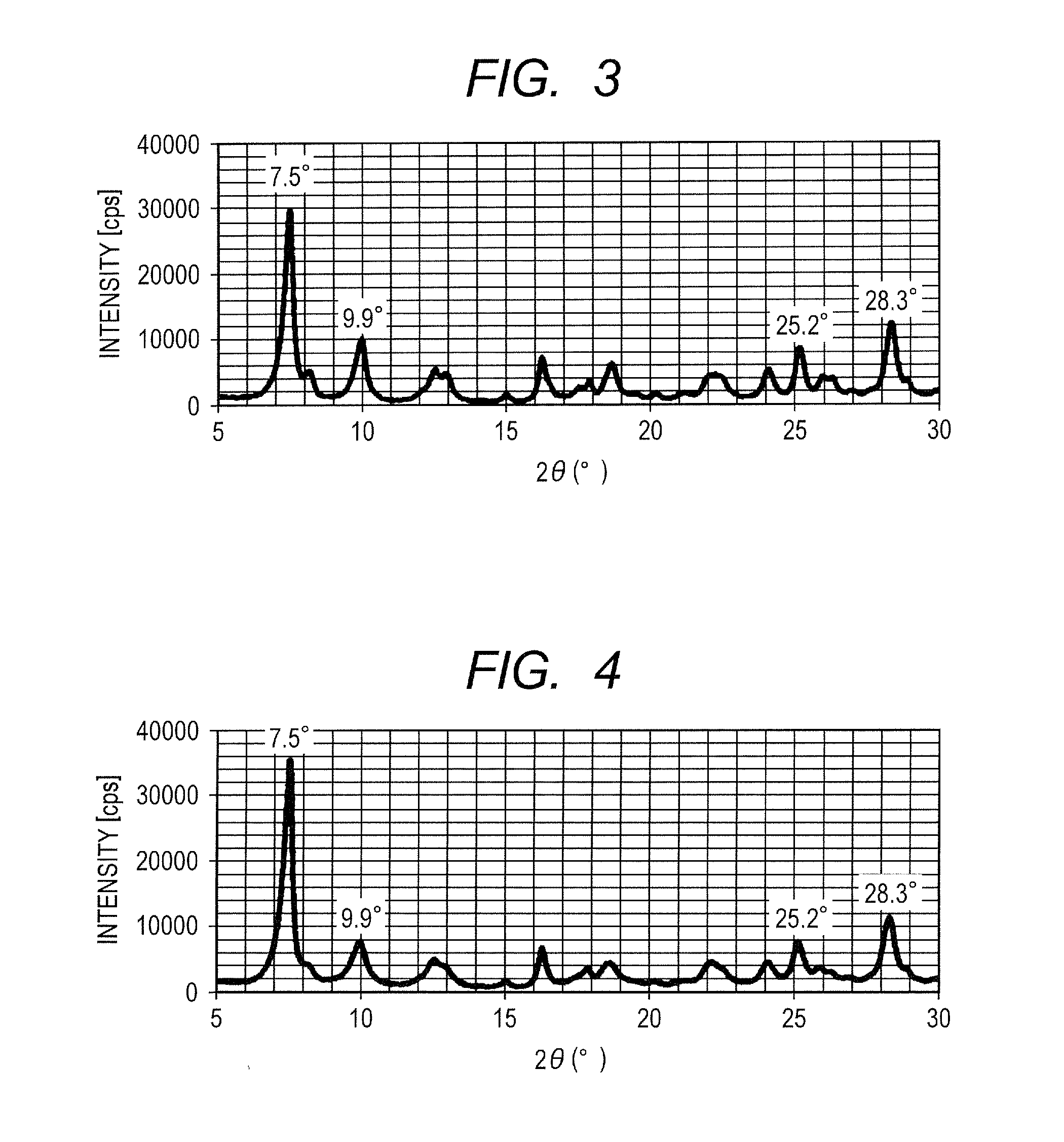
[0079]A hydroxygallium phthalocyanine crystal in an amount of 0.42 parts was obtained by the same treatment as in Example 1-1, except that the milling treatment time was changed from 1000 hours to 2000 hours in Example 1-1. The powder X-ray diffraction pattern of the produced crystal was the same as in Example 1-1. The angle and the intensity of the peak emerging at Bragg angle 2θ of 9.9°±0.2°, the intensity on a side 2.8° wider from the peak angle, and the ratio of the intensity are described in Table 1.
[0080]It was confirmed that 0.80% by mass N,N-dimethylformamide is contained relative to phthalocyanine in the phthalocyanine crystal by the NMR measurement.
example 1-3
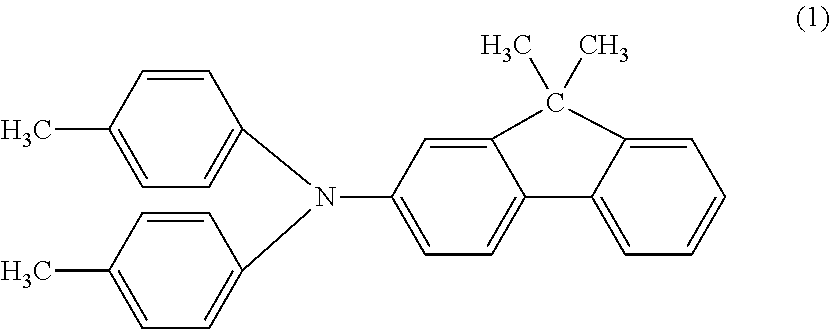
[0081]A hydroxygallium phthalocyanine crystal in an amount of 0.44 parts was obtained by the same treatment as in Example 1-1, except that the milling treatment time was changed from 1000 hours to 500 hours in Example 1-1. The powder X-ray diffraction pattern of the produced crystal was the same as in Example 1-1. The angle and the intensity of the peak emerging at Bragg angle 2θ of 9.9°±0.2°, the intensity on a side 2.8° wider from the peak angle, and the ratio of the intensity are described in Table 1.
[0082]It was confirmed that 1.23% by mass N,N-dimethylformamide is contained relative to phthalocyanine in the phthalocyanine crystal by the NMR measurement.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bragg angles 2θ | aaaaa | aaaaa |
| Bragg angles 2θ | aaaaa | aaaaa |
| Bragg angles 2θ | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com