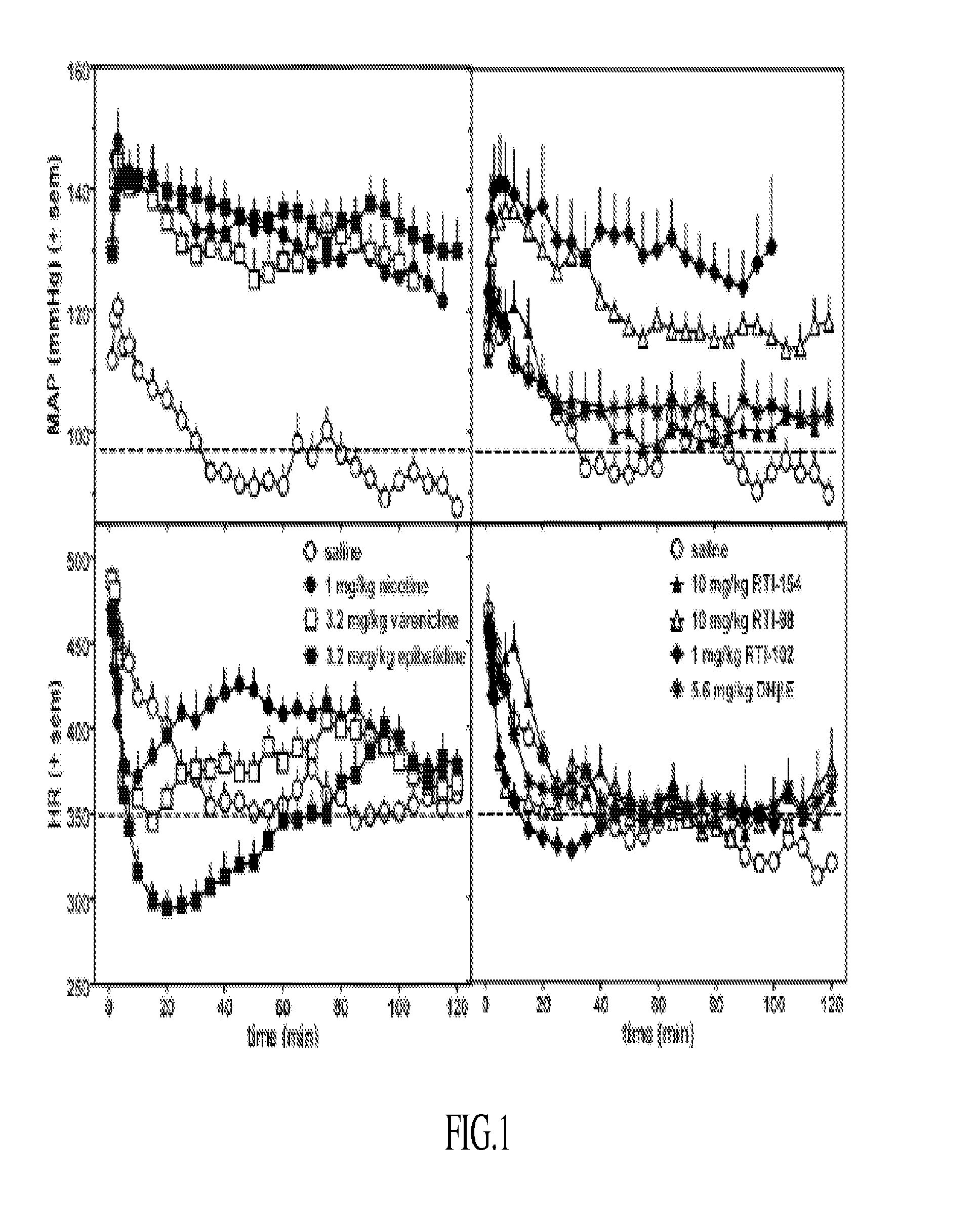
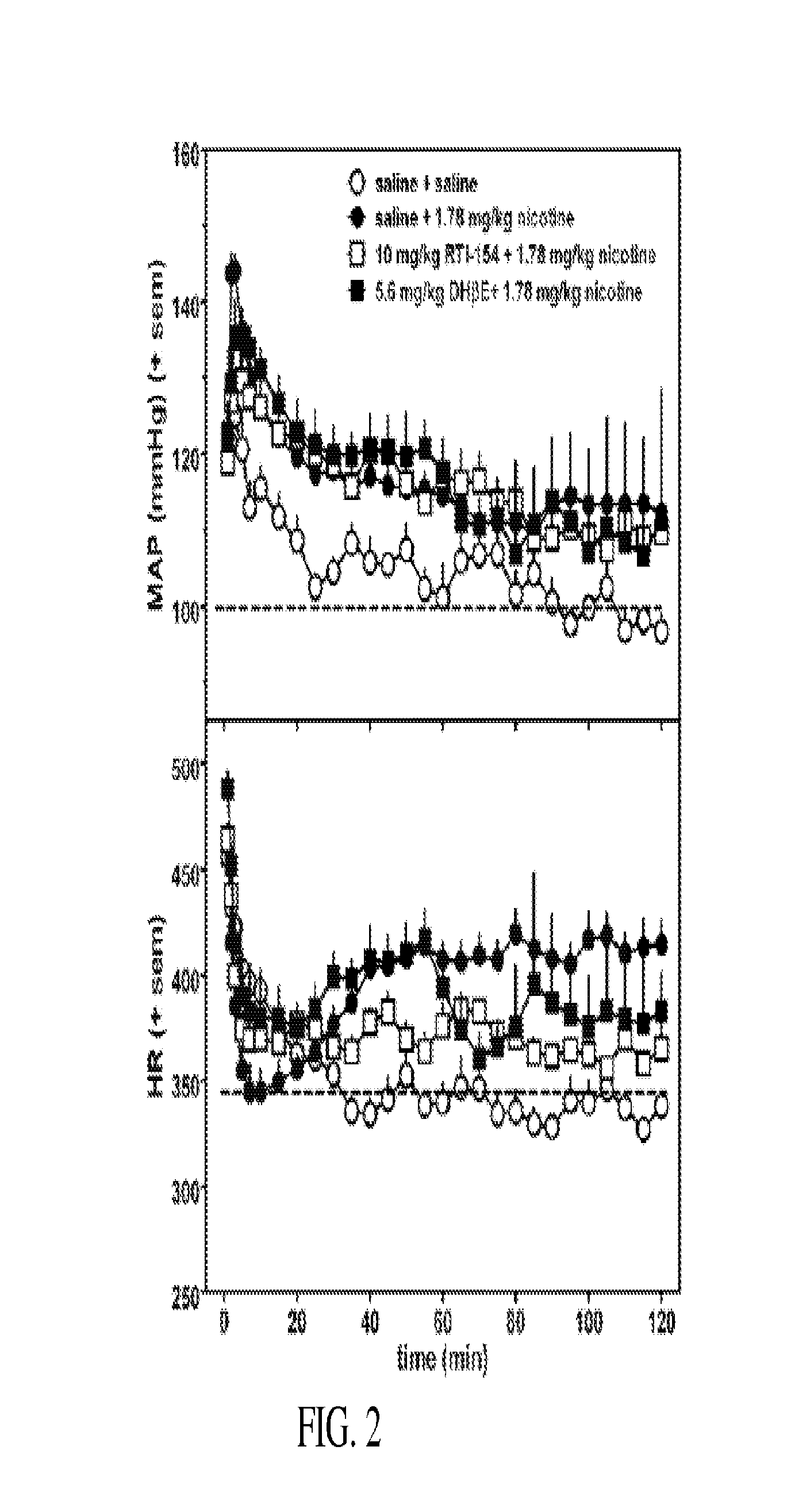
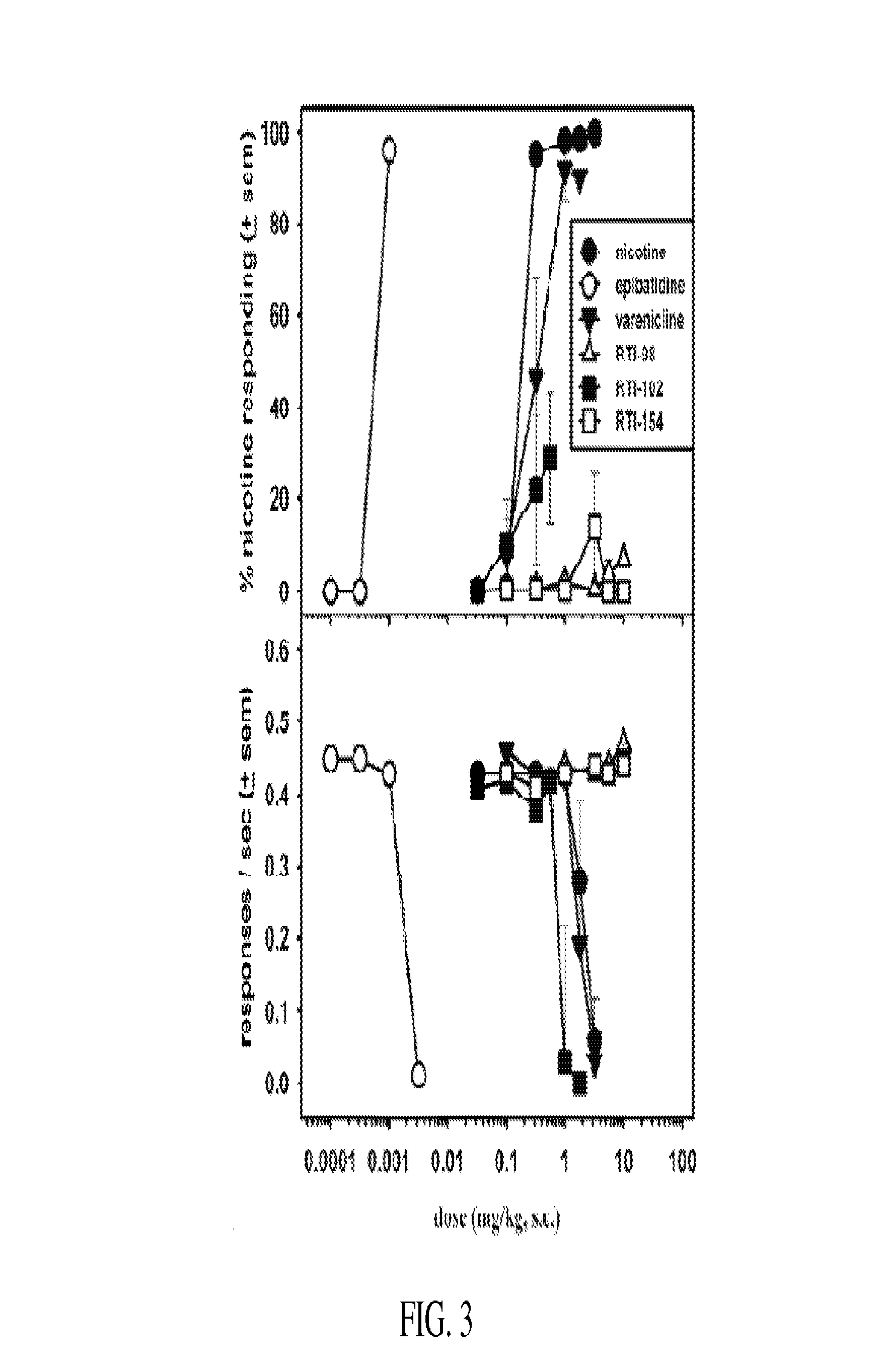
Nicotinic receptor compounds
a technology of nicotinic receptors and compounds, applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems of individuals quitting smoking, unable to quit smoking tobacco products, and extremely difficult to provide therapeutic treatment for smoking addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 2′-Fluoro-3′-(4-substituted-phenyl)deschloroepibatidine analogues
[0061]
X═SO2NH2 (RTI-7527-192), SO2CF3 (RTI-7527-193), CF3 (RTI-7527-153), SO2CH3 (RTI-7527-154), CN(RTI-7527-155)
[0062]The steps in the synthesis of 2′-fluoro-3′-(4-benzenesulfonamide), 2′-fluoro-3′-(4-trifluoromethanesulfonyl phenyl), 2′-fluoro-3′-(4-trifluoromethylphenyl), 2′-fluoro-3′-(4-methanesulfonylphenyl), and 2′-fluoro-3′-(4-cyanophenyl) deschloroepibatidines were carried out as represented in Schemes 1-4 below.
[0063]Heck-cross coupling of olefin 1, followed by bromination of 3 and fluorination of 4 via the Sandmeyer reactions were all performed to provide the 2′-fluoro-3′-bromo compound 5. Boc re-protection was achieved by stirring 5 in the presence of Boc anhydride, Et3N and DMAP in THF as the solvent to furnish compound 6. Suzuki-Miyaura borylation of 2′-fluoro-3′-bromo deschloroepibatidine (6) was performed through a microwave assisted cross-coupling of 6 with bis(pinacolato)diboron in the pre...
example 2
Experimental Procedures
7-tert-Butoxycarbonyl-2-exo-(2′-fluoro-3′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5′-pyridinyl)-7-azabicyclo[2.2.1]heptane (7)
[0070]
[0071]In a microwave vial was placed 7-tert-butoxycarbonyl-2-exo-(2′-fluoro-3′-bromo-5′-pyridinyl)-7-azabicyclo[2.2.1]heptane (6) (214 mg, 0.578 mmol, 1.0 equiv), bis(pinacolato)diboron (176 mg, 0.694 mmol, 1.2 equiv), KOAc (170 mg, 1.73 mmol, 3.0 equiv), Pd(dppf)Cl2 (21 mg, 0.0289 mmol, 5 mol %) and anhydrous 1,4-dioxane (3 mL). The mixture was degassed through bubbling nitrogen for 20 min and then was irradiated in a microwave at 140° C. for 15 min. After cooling to room temperature, the mixture was diluted with EtOAc, filtered through a plug of Celite and anhydrous Na2SO4 and concentrated in vacuo. The resultant residue was purified by flash chromatograph through an ISCO column (EtOAc-hexanes) to provide 200 mg (83%) of 7 as a colorless oil.
[0072]1H NMR (300 MHz, CDCl3) δ (ppm) 1.26 (s, 12H), 1.44 (s, 9H), 1.60-1.53 (m, 2...
example 3
Synthesis of RTI-7527-168 and RTI-7257-169
[0104]
[0105]Reagents and conditions: (a) 70% HF-pyridine, NaNO2 (b) Pd(PPh3)4, carbamoyl phenyl boronic acid, K2CO3, 1,4-dioxane, H2O, reflux, 24 h (c) HCl in Ether.
2-exo-[2′-Fluoro-3′-(4-aminocarbonylphenyl)-5′-pyridinyl]-7-azabicyclo[2.2.1]heptane (18)
[0106]A solution of 2-exo-[2′-Fluoro-3′-bromo-5′-pyridinyl]-7-azabicyclo[2.2.1]heptane (5) (178 mg, 0.66 mmol), 4-carbamoylphenyl boronic acid (130 mg, 0.79 mmol), Pd(PPh3)4 (38 mg, 5 mol %), and K2CO3 (182 mg, 1.31 mmol) in 1,4-dioxane (5 mL) and H2O (0.8 mL) in a sealed tube was degassed through bubbling N2 for 20 min then heated at 100° C. for 20 h. After cooling to room temperature, the solvent was removed in vacuo and the residue was re-dissolved in EtOAc. Water (20 mL) was added and the organic product extracted with EtOAc (3×30 mL). The combined organic layers were dried over anhydrous MgSO4, filtered through Celite and concentrated in vacuo. The crude residue was purified on silica ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com