Compositions and methods for improving rebaudioside x solubility
a technology of rebaudioside and solubility, which is applied in the field of crystallized forms and amorphous rebaudiosides, can solve the problems of poor aqueous solubility and dissolution qualities of rebaudioside x obtained from stevia rebaudiana, and achieve the effect of improving solubility properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of Reb X from Stevia rebaudiana Bertoni Plant Leaves
[0553]Two kg of Stevia rebaudiana Bertoni plant leaves were dried at 45° C. to an 8.0% moisture content and ground to 10-20 mm particles. The content of different glycosides in the leaves was as follows: Stevioside—2.55%, Reb A—7.78%, Reb B—0.01%, Reb C—1.04%, Reb D—0.21%, Reb F—0.14%, Reb X—0.10% Dulcoside A—0.05%, and Steviolbioside—0.05%. The dried material was loaded into a continuous extractor and the extraction was carried out with 40.0 L of water at a pH of 6.5 at 40° C. for 160 min. The filtrate was collected and subjected to chemical treatment. Calcium oxide in the amount of 400 g was added to the filtrate to adjust the pH within the range of 8.5-9.0, and the mixture was maintained for 15 min with slow agitation. Then, the pH was adjusted to around 3.0 by adding 600 g of FeCl3 and the mixture was maintained for 15 min with slow agitation. A small amount of calcium oxide was further added to adjust the pH to 8....
example 2
Structural Elucidation of Rebaudioside X
[0563]HRMS: HRMS (High Resolution Mass Spectrum) data was generated with a Waters Premier Quadrupole Time-of-Flight (Q-TOF) mass spectrometer equipped with an electrospray ionization source operated in the positive-ion mode. Samples were diluted and eluted with a gradient of 2:2:1 methanol:acetonitrile:water and introduced 50 μL via infusion using the onboard syringe pump
[0564]NMR: The sample was dissolved in deuterated pyridine (C5D5N) and NMR spectra were acquired on Varian Unity Plus 600 MHz instruments using standard pulse sequences. The chemical shifts are given in δ (ppm), and coupling constants are reported in Hz.
[0565]The complete 1H and 13C NMR spectral assignments for the diterpene glycoside rebaudioside X determined on the basis of 1D (1H and 13C) and 2D (COSY, HMQC and HMBC) NMR as well as high resolution mass spectroscopic data:
[0566]Discussion
[0567]The molecular formula was deduced as C56H90O33 on the basis of its positive high r...
example 3
Preparation and Characterization of Form A Rebaudioside X
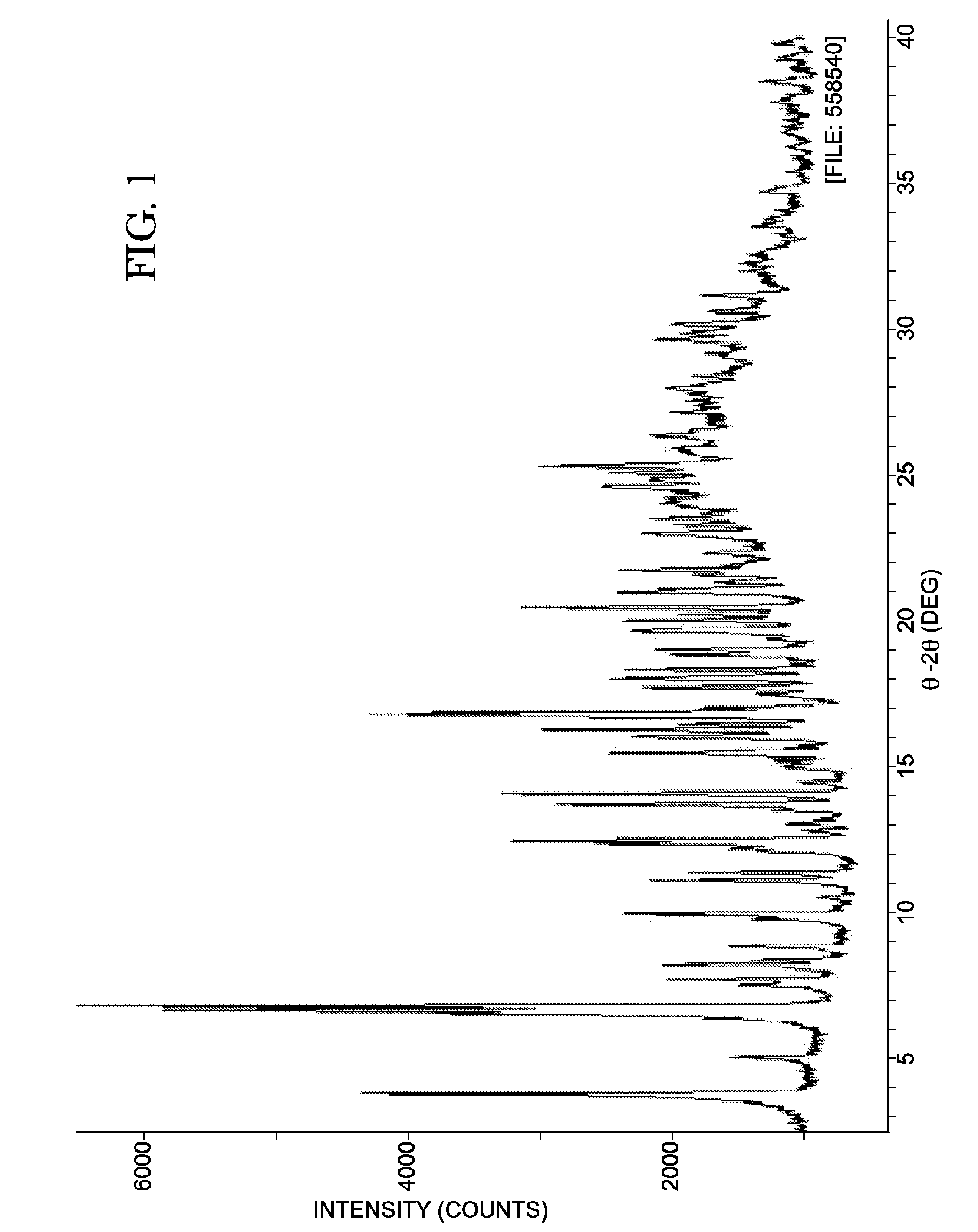
[0573]X-ray amorphous Rebaudioside X was added to a 1:1 mixture of methanol and water to provide a slurry. The slurry was stirred at room temperature overnight. The X-ray diffraction pattern of the Rebaudioside X obtained is shown in FIG. 1. Form A was successfully indexed, indicating that the sample is composed primarily of a single crystalline phase. Prominent peaks are provided below:
TABLE 1Form A Rebaudioside X Prominent XPRD°2Θd space (Å)Intensity (%) 3.76 ± 0.2023.489 ± 1.319 67 6.50 ± 0.2013.594 ± 0.431 58 6.62 ± 0.2013.354 ± 0.416 89 6.79 ± 0.2013.025 ± 0.395 100 9.93 ± 0.208.909 ± 0.1833612.33 ± 0.207.176 ± 0.1184012.45 ± 0.207.109 ± 0.1164913.69 ± 0.206.469 ± 0.0954414.06 ± 0.206.301 ± 0.0905015.44 ± 0.205.738 ± 0.0753716.25 ± 0.205.456 ± 0.0684616.80 ± 0.205.278 ± 0.0636620.44 ± 0.204.345 ± 0.04248
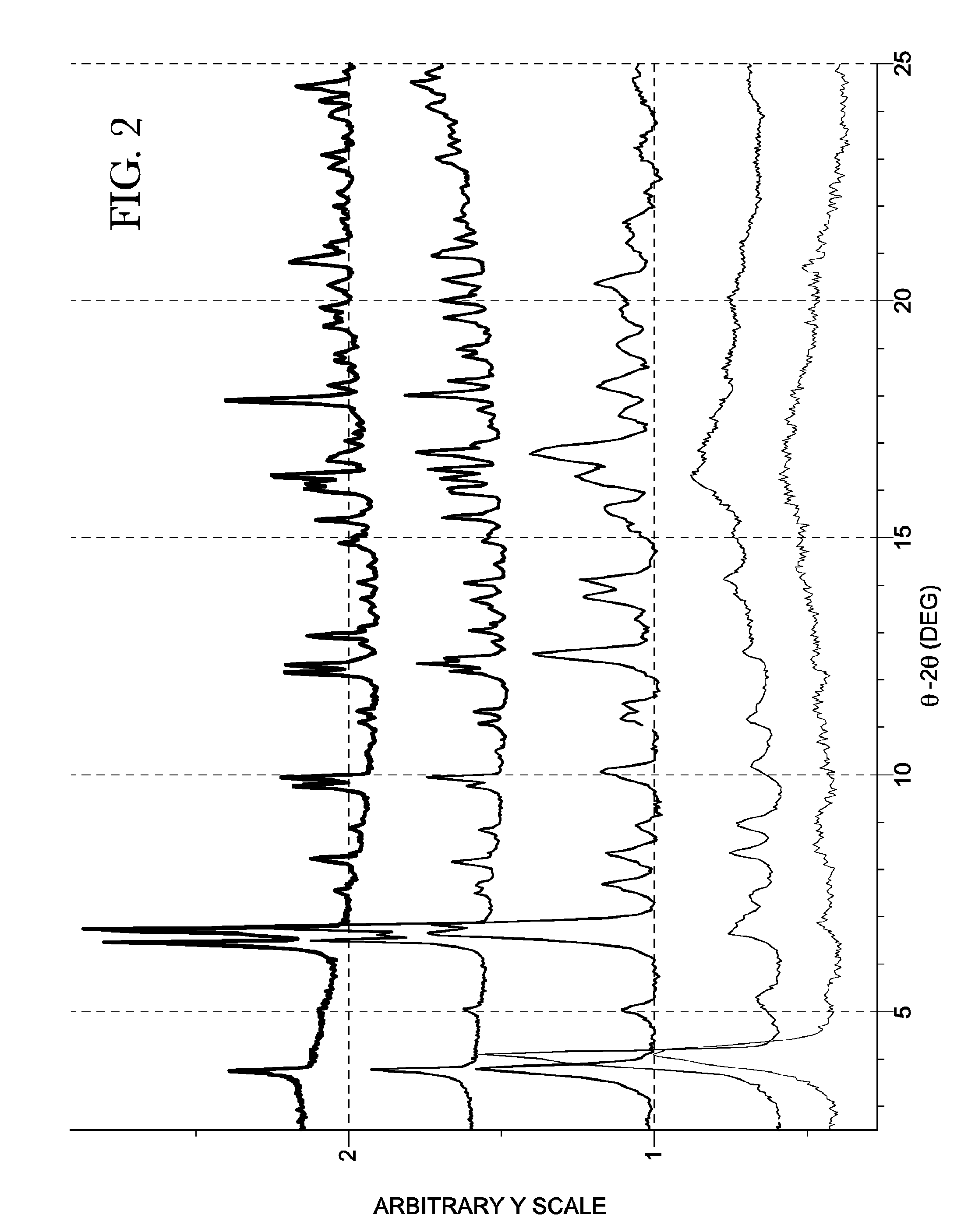
[0574]Non-systematic peak shifts between the X-ray diffraction patterns of Form A are observed (FIG. 2) and are likely d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com