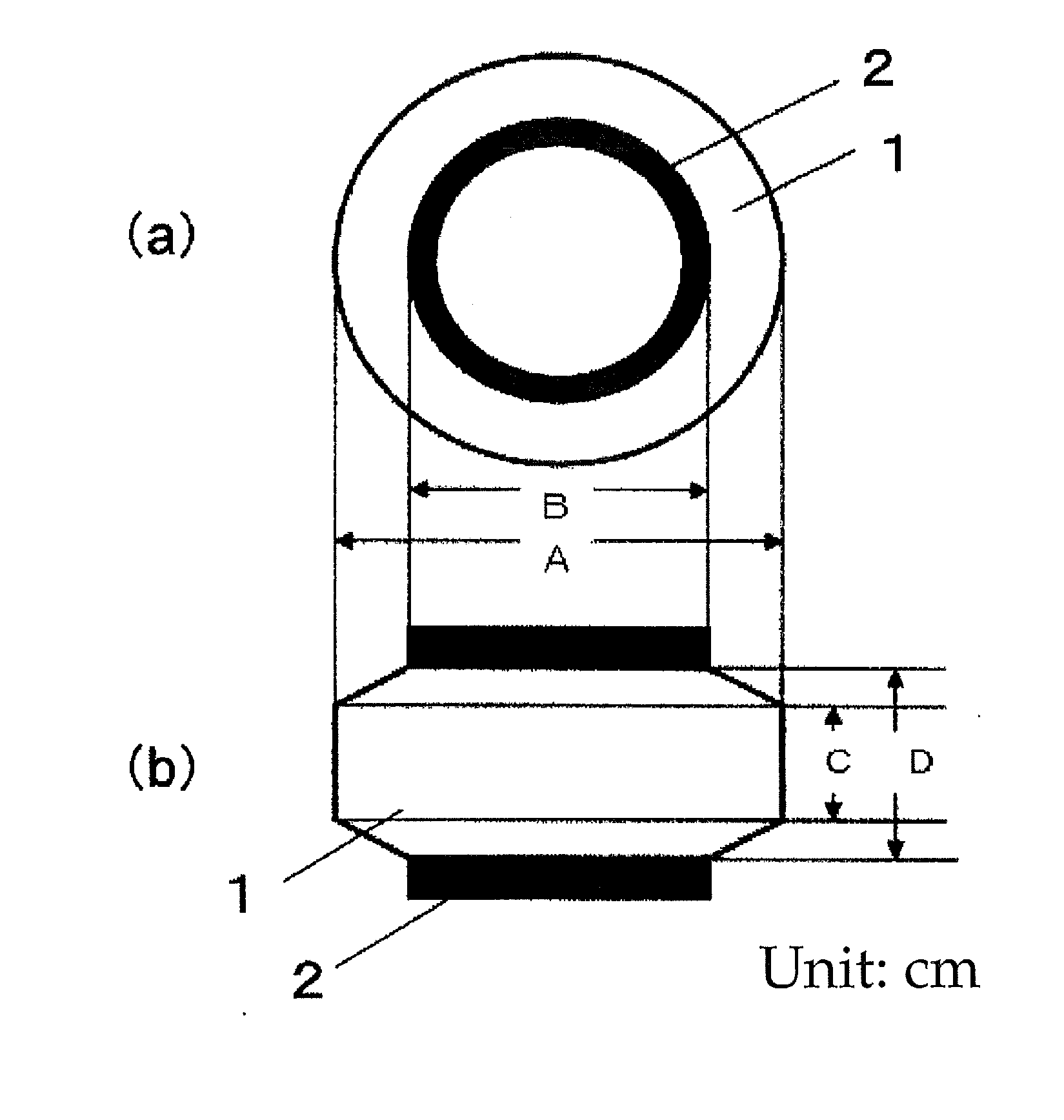
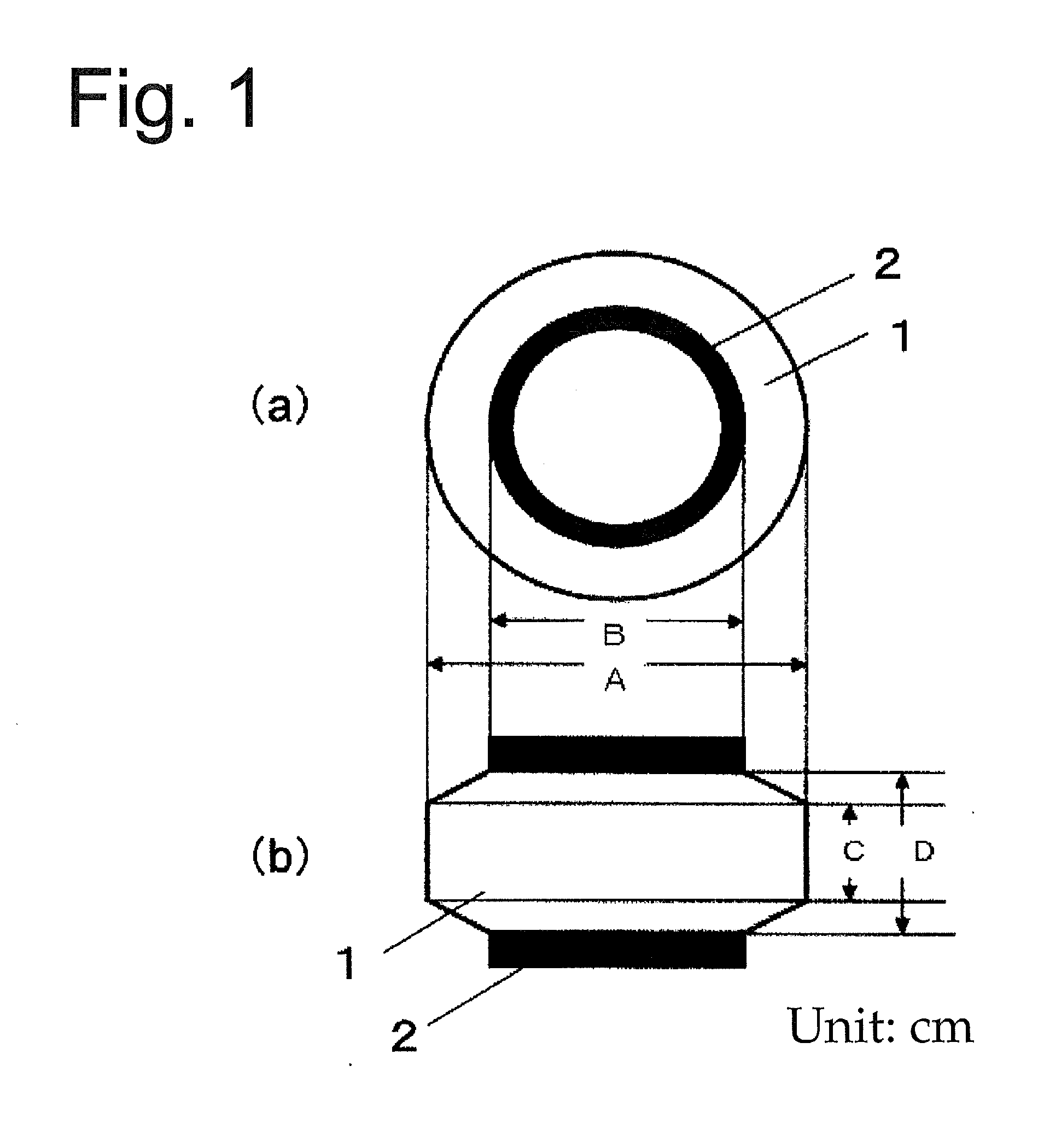
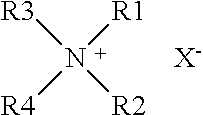
Elastic polyurethane thread and manufacturing method thereof
a technology of polyurethane and manufacturing method, which is applied in the direction of monocomponent polyurethane artificial filament, dyeing process, layered products, etc., can solve the problems of yellowing completely solved, the value of the product being greatly reduced, and the inability to say, etc., to achieve excellent stretch ability and deodorant, antibacterial, color fastness properties, and antibacterial effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
[0081]This invention will be explained in more detail by using working examples.
[0082]Strengths, stress relaxation percentages, permanent strain rates, and ductility of the elastic polyurethane thread:
The strengths, stress relaxation percentages, permanent strain rates, and ductility of the elastic polyurethane thread were measured by performing tensile tests with an Instron 4502 tensile tester.
[0083]They are defined below.
[0084]A 5 cm (L1) sample was stretched 300% 5 times at a tensile speed of 50 cm / min. The stress at the 5th time was taken to be (G1). Next, a 300% elongation was held for 30 seconds. The stress after it was held for 30 seconds was taken to be (G2). Next, the length of the sample when the elongation was repeated and the stress became 0 was taken to be (L2). Furthermore, the sample thread was elongated a 6th time until it broke. The stress at the time of the break was taken to be (G3) and the length of the sample thread when it broke was taken to be (L3).
[0085]The p...
working example 1
[0105]PTMG with a molecular weight of 1800 and MDI were reacted for 2 hours at 90° C. in the molar ratio 1:1.58 to produce a prepolymer with isocyanate ends, after which it was dissolved in DMAc at 35 wt % to prepare a prepolymer solution. Moreover, an amine solution was prepared by mixing ethylenediamine and 1,2-propanediamine as chain extenders and diethylamine as a chain terminator (end sequestering agent) in the proportions of 10:2:1 amino end group concentrations and dissolving this mixture in DMAc at 35 wt %. The prepolymer solution and the amine solution were mixed while stirring so that the molar ratio of the isocyanate end groups and amine end groups became 1:1.02, to prepare a DMAC solution (concentration 35 wt %) of a polyurethane urea polymer. Next, as an antioxidant, a polyurethane solution (Dupont Co. “Methacrol”™ 2462) produced by a reaction of t-butyldiethanolamine and methylene-bis(4-cyclohexyl isocyanate) and a condensation polymer of p-cresol and divinylbenzene (D...
working example 2
[0110]The polymer solutions A1, B1, and C1 were uniformly mixed at 97.98 wt %, 2 wt %, and 0.02 wt % to make the spinning dope D2. This was dry-spun in the same manner as in Working Example 1 to make 200 g of a wound polyurethane 22 desitex, 2 filament thread with a zirconium phosphate content of 2 wt %.
[0111]The evaluation results are shown in Tables 3, 4, and 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon number | aaaaa | aaaaa |
| average primary particle diameters | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com