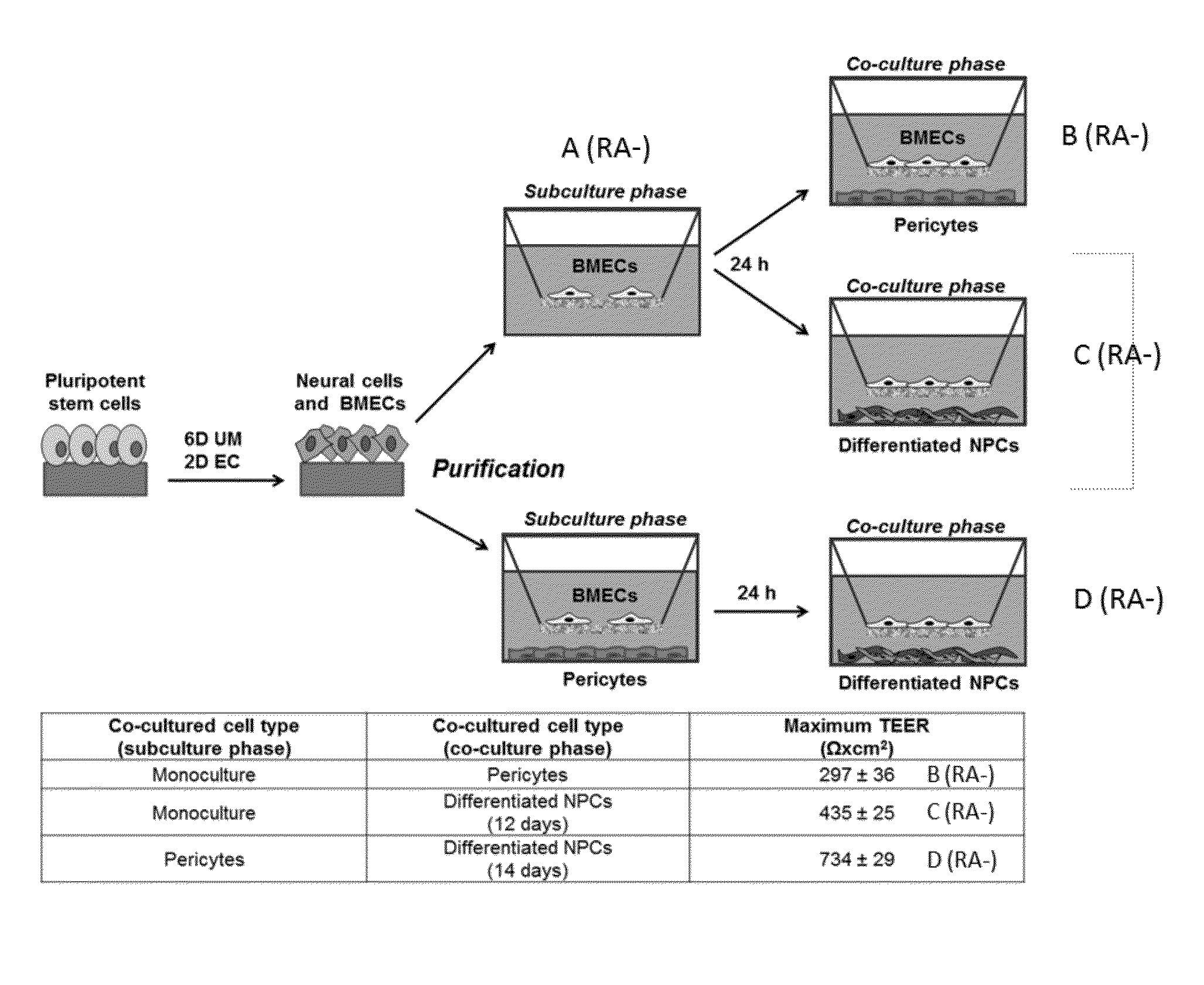
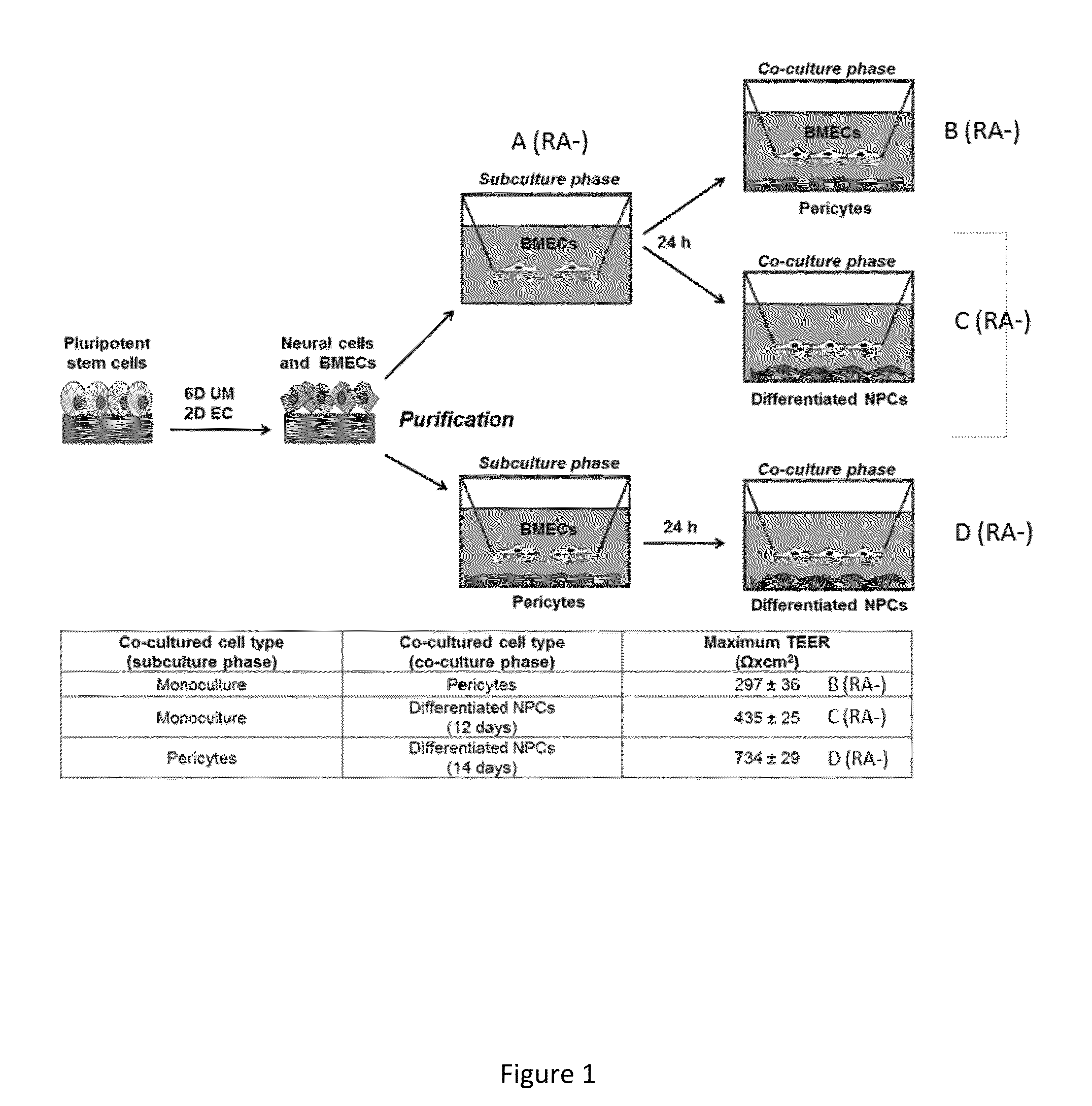
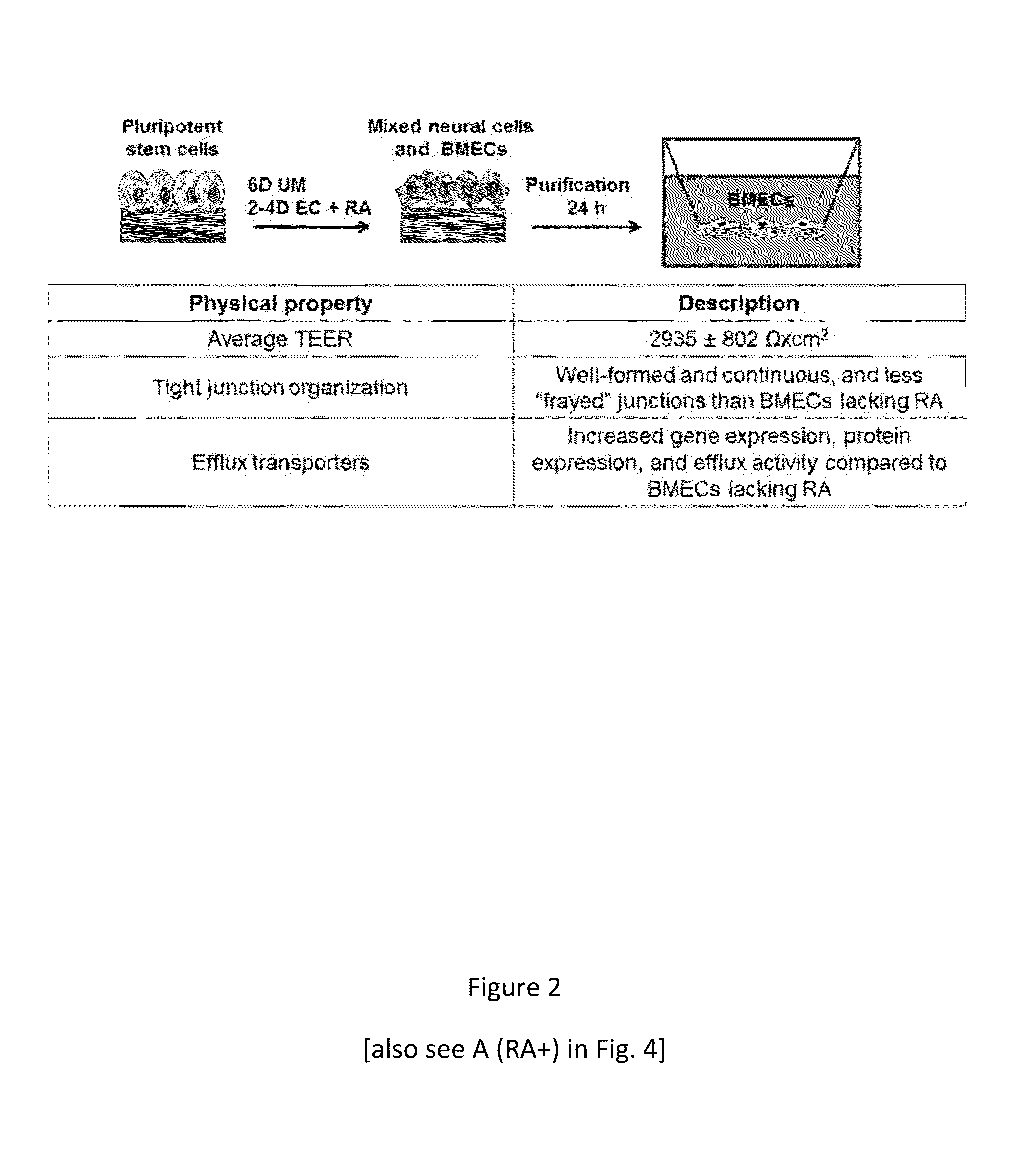
Retinoic Acid Enhanced Human Stem Cell Derived Blood Brain Barrier Model
a human stem cell and retinoic acid technology, applied in the field of retinoic acid enhanced human stem cell derived blood brain barrier model, can solve the problems of inability to screen brain-penetrating therapeutics in vivo, difficult and time-consuming in vivo studies involving bbb development and regulation, and inability to scale up large-scale library screens. source of material for large-scale screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
[0088]hPSC Differentiation to BMECs.
[0089]IMR90-4 and DF19-9-11T hiPSCs and H9 hESCs were maintained between passages 26-42 on MATRIGEL (BD Biosciences) in mTeSR1™ medium (STEMCELL Technologies) or on irradiated mouse embryonic fibroblasts (MEFs) in standard unconditioned medium (Dulbecco's Modified Eagle's Medium [DMEM] / Ham's F12 containing 20% Knockout Serum Replacer (Invitrogen), 1×MEM nonessential amino acids (Invitrogen), 1 mM L-glutamine (Sigma), 0.1 mM β-mercaptoethanol (Sigma), and human basic fibroblast growth factor (bFGF; 100 ng / mL for hiPSCs and 4 ng / mL for hESCs; Waisman Clinical Biomanufacturing Facility, University of Wisconsin-Madison)). Prior to differentiation, cells were passaged onto Matrigel (BD Biosciences) in mTeSR1 medium (STEMCELL Technologies). After 2-3 days in mTeSR1, medium was switched to unconditioned medium (UM) lacking bFGF for 6 days. Human endothelial serum-free medium (hESFM; Invitrogen) supplemented with 20 ng / mL bFGF (R&D Sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com