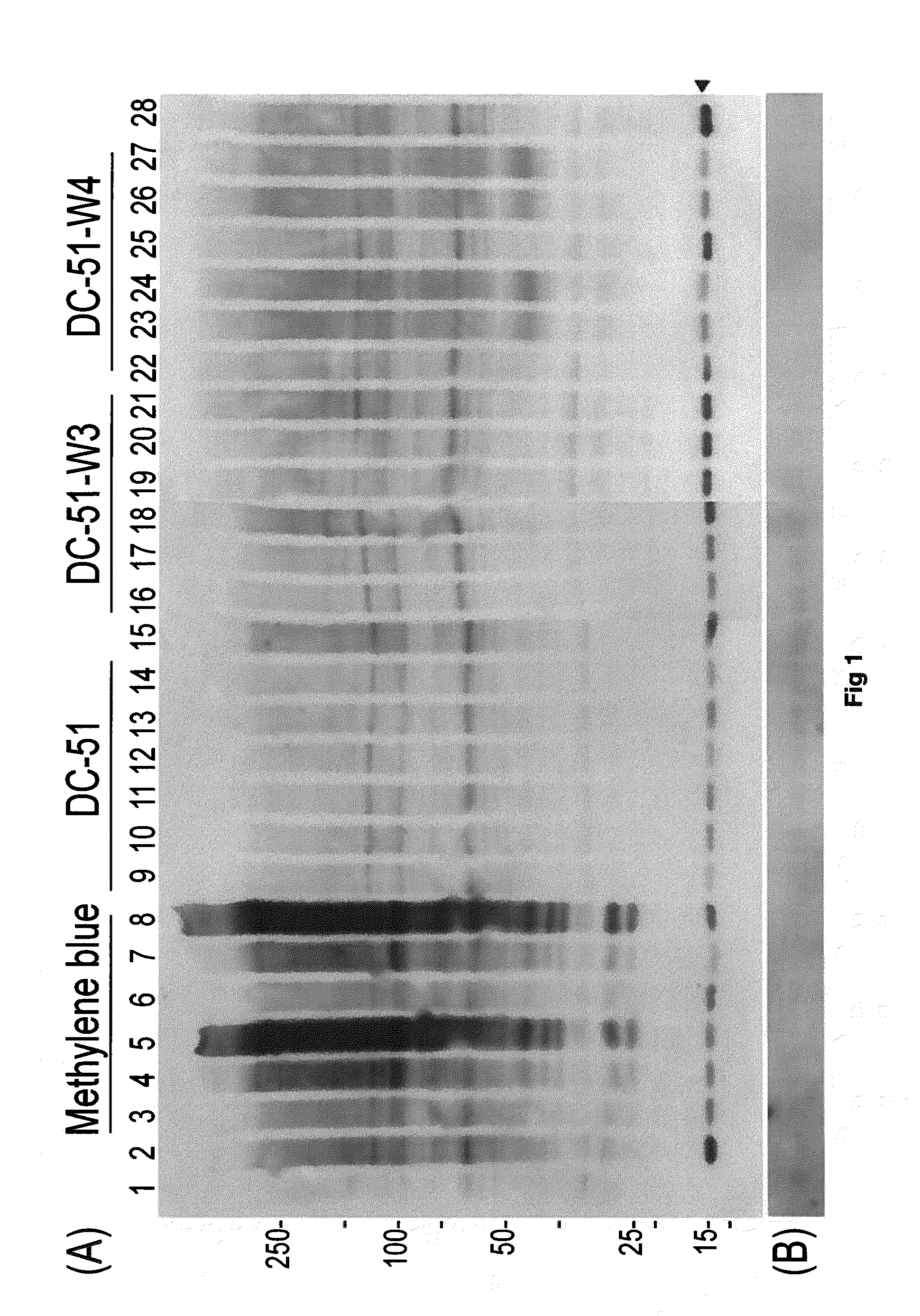
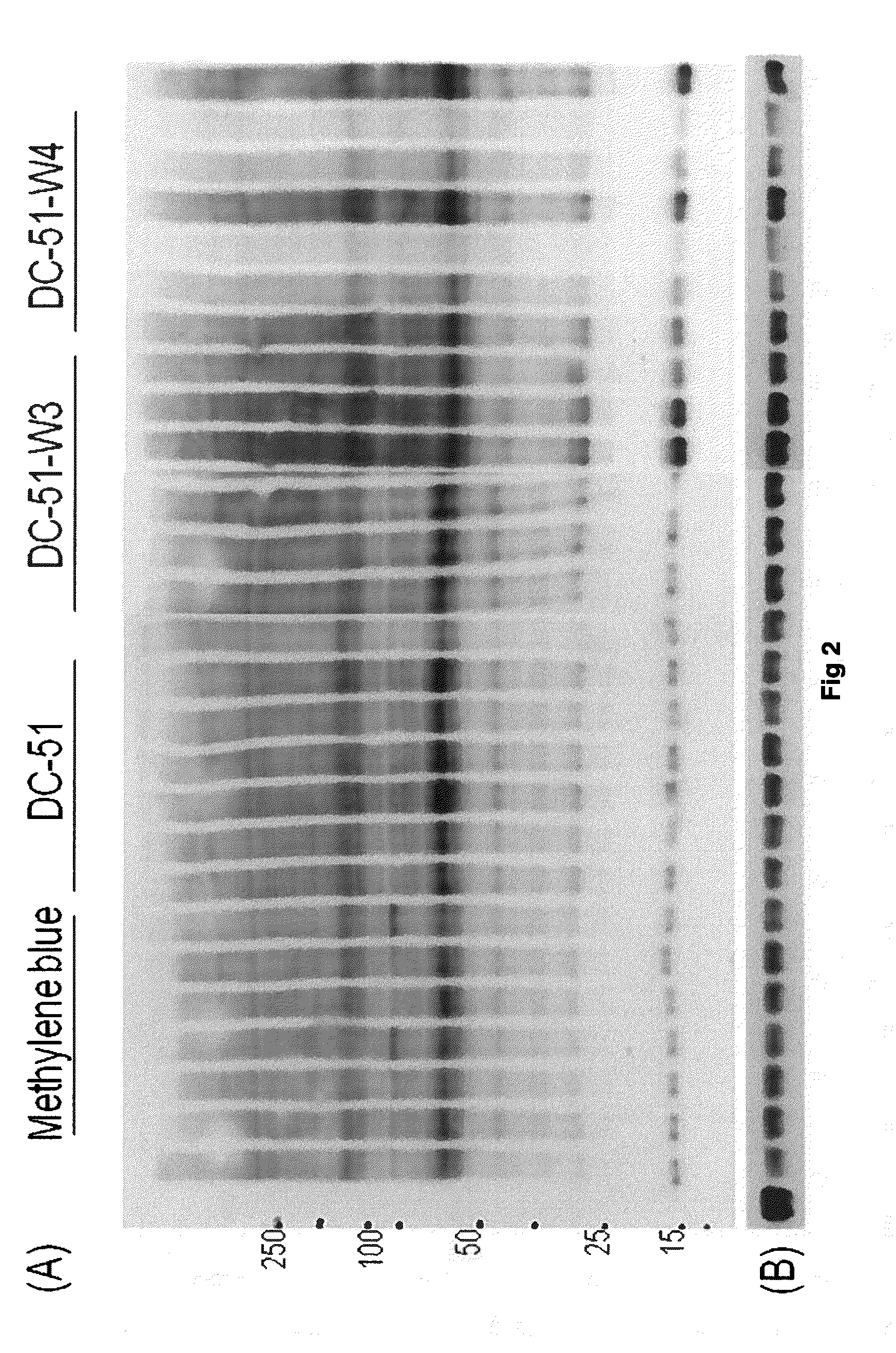
Compounds and compositions for use as modulators of tau aggregation and alleviation of tauopathies
a tauopathies and modulator technology, applied in the direction of heterocyclic compound active ingredients, biocide, amide active ingredients, etc., can solve the problems of neurotoxicity of protein aggregates and overstabilization of microtubules, and achieve the effect of reducing tauopathies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Compounds of this Invention are Potent Disrupters of Tau Aggregates
[0074]The compounds set out above were found to be potent in the dissolution / disruption / inhibition of Tau tangles or Tau aggregates. In a set of studies, the efficacy of the compounds to cause a dissolution / disassembly / disruption of pre-formed Tau aggregates was analyzed.
Part A—Thioflavin T Fluorometry Data
[0075]Thioflavin T fluorometry was used in this study to determine the effects of the compounds compared to a negative control peptide. Thioflavin T binds specifically to aggregated Tau or Tau tangles, and this binding produces a fluorescence enhancement at 485 nm that is directly proportional to the amount of aggregated Tau. The higher the fluorescence, the greater the amount of aggregated Tau.
[0076]In this study, Tau-441 was pre-fibrillized or aggregated by combining with Heparin (SIGMA) at 1:1 wt / wt, then incubation at 37° C. and shaking at 1400 rpm for 8 days. Following the pre-fibrillization, 30 μg of aggregat...
example 2
Cloning of Tau Repeat Domains (TauRD) into a Tetracycline-Inducible Mammalian Expression Vector
[0078]Total RNA was isolated from human adult non-demented frontal tissues obtained at autopsy from the University of Washington ADRC Brain Bank and immediately frozen at 80° C. Single stranded cDNA was synthesized using M-MLV Reverse Transcriptase (Invitrogen; Carlsbad, Calif., USA) and random priming with hexameric primers (Invitrogen). All other primers used were also synthesized by Invitrogen. Tetracycline-inducible mammalian expression constructs, pcDNA4 / TO-TauRD, were generated by insertion of cDNA fragments encoding the human tau repeat domain (TauRD; amino acid residues 244-372 in REFSEQ mRNA ENST00000351559) into pcDNA™4 / TO vector (Invitrogen). The vector allows tetracycline-regulated expression of the gene of interest in mammalian host cells when a pcDNA™6 / TR construct (Invitrogen) is also co-expressed.
[0079]The wild type TauRD (TauRDWT) cDNA insert was amplified from human brain...
example 3
Generation of Stable Transfected Inducible TauRD Cell Lines
[0080]Tetracycline-inducible cell lines stably transfected with pcDNA4 / TO-TauRDWT, pcDNA4 / TO-TauRDΔK280, pcDNA4 / TO-TauRDP301S, and pcDNA4 / TO-TauRDP301L were generated to assess TauRD aggregation in cell culture. TREx™-293 cells (Invitrogen; R710-07), modified human embryonic kidney 293 cells (ATCC; CRL-1573) by stable transfection of pcDNA™6 / TR, were employed to generate the TauRD stable cell lines. Both parental cells (TREx™-293 cells) and their derivatives (TREx293-TauRD) (see below) were maintained in culture media supplemented with 5 μg / ml blasticidin for selection of pcDNA6 / TR-containing cells. Cells were routinely cultured in a regular growth media (RGM) that contained Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum, penicillin (60 units / mL), streptomycin (60 μg / mL) and blasticidin (5) at 37° C. in a cell culture incubator supplemented with 5% CO2.
[0081]To generate stable c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com