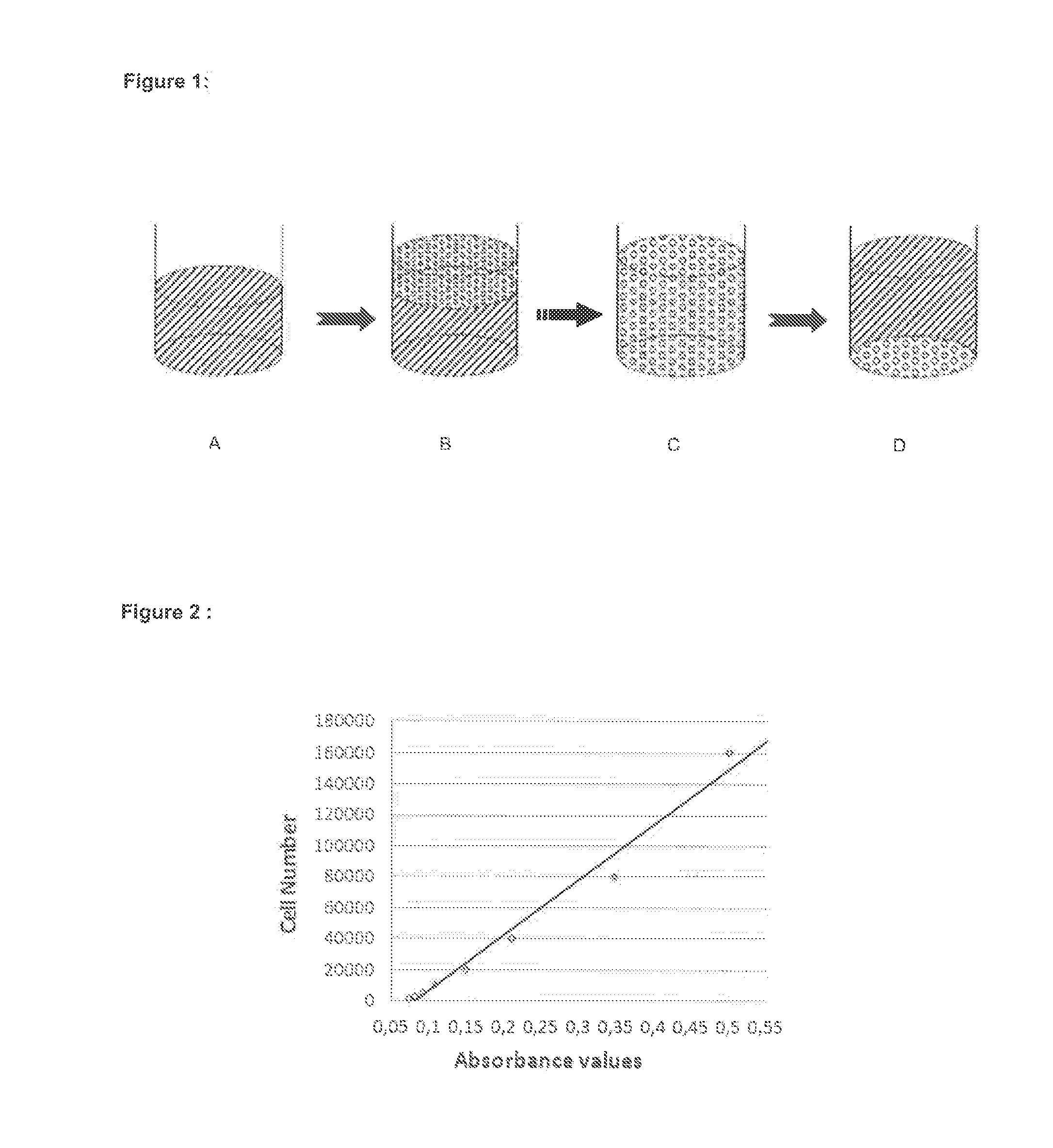
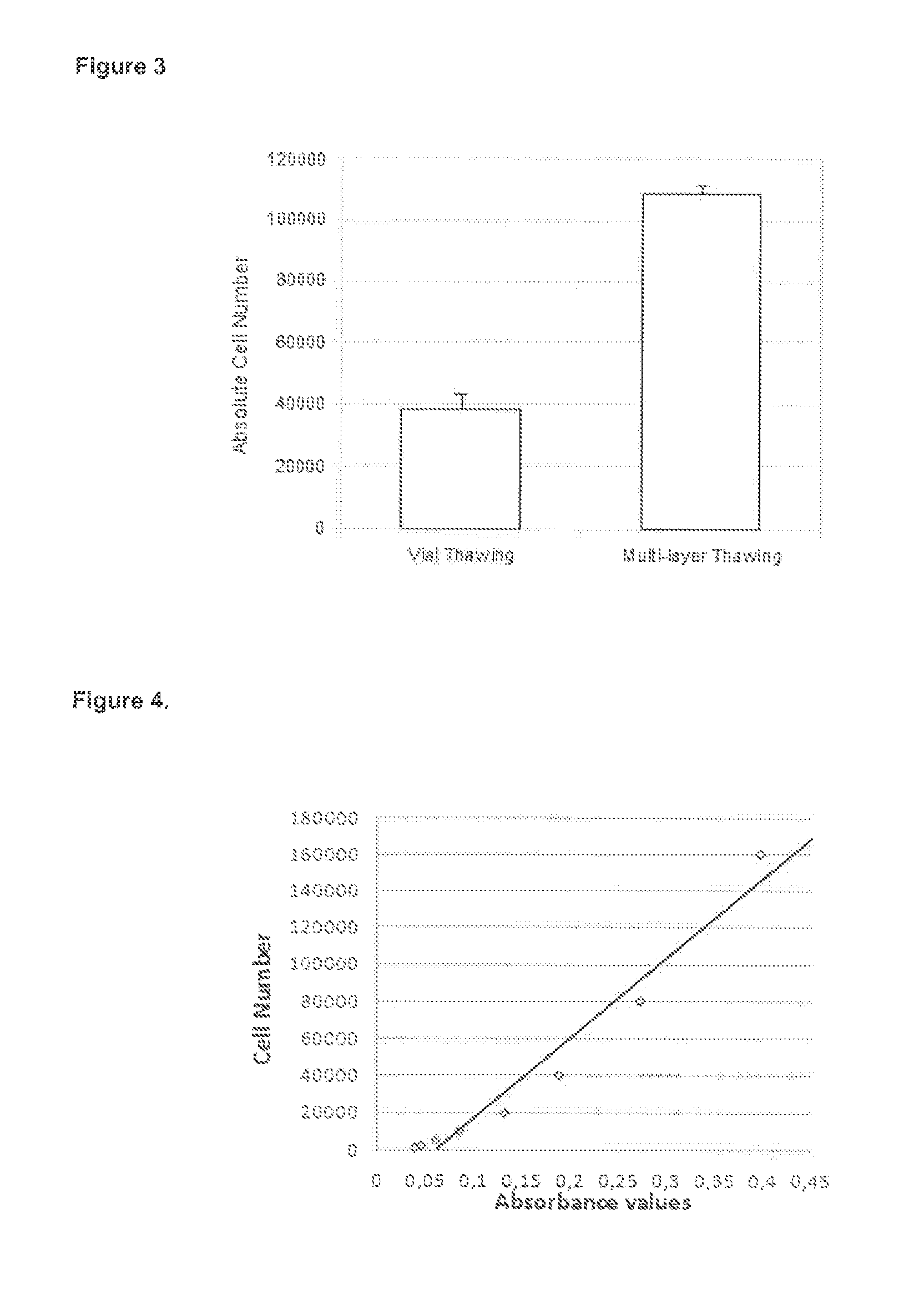
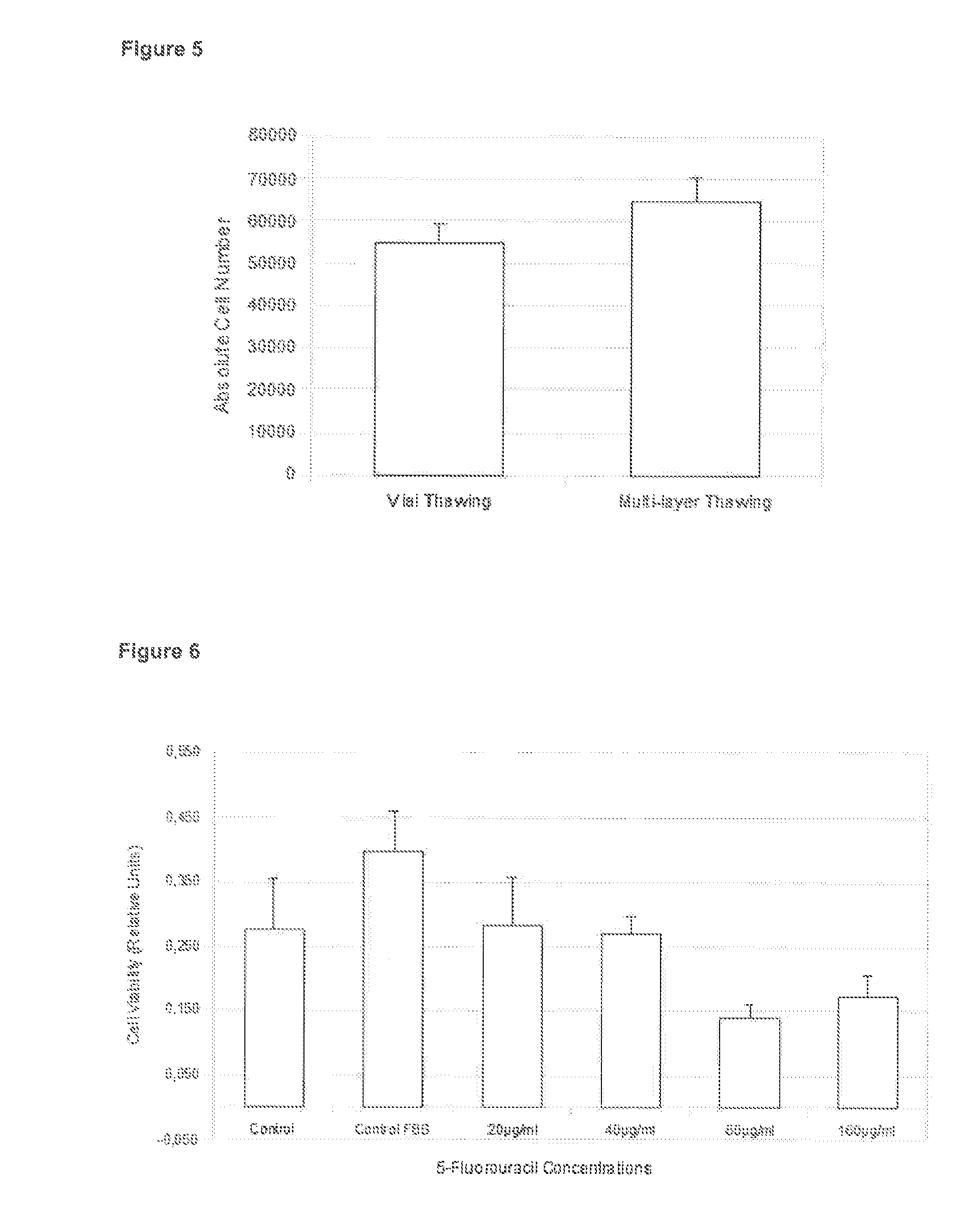
Method of freezing cells
a cell culture and cell technology, applied in the field of multi-layer cell freezing vials, can solve the problems of microbial contamination, laborious and time-consuming cell culture methods, and low cell number, and achieve the effect of reducing the number of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Two-Layer Cell Freezing / Thawing Method on HT29 Colon Carcinoma Cells
[0054]HT29 Cells (ATCC, LGC Standards) cultured in endotoxin-free McCoy's 5A complete culture medium supplemented with 10% FCS (Gibco), 100 μg / ml penicillin and 100 IU / ml streptomycin (Sigma-Aldrich) were grown in flasks until they were 80% confluent, and than harvested. Harvesting took place by incubating cells with 5 ml of PBS containing 0.05% EDTA for 2 min, and final detachment was achieved by incubation in 5 ml of PBS with 0.1% trypsin and 0.05% EDTA for another 5 min. Cells were centrifuged at 400×G for 5 min. Then, a total of 4.8 ml of a solution at 4° C. containing 80,000 HT29 cells / 50 μl of freezing 80% Mc Coy's, 10% FCS, 10% DMSO (AppliChem) medium was prepared (cell density determined by haemocytometer), forming the no called freezing solution. Besides, 100 μl of complete culture medium was disposed in each cell of a 96-well plate and frozen down to −80° C. Once frozen, the plate is recovered and 50 μl / we...
example 2
Two-Layer Cell Freezing / Thawing Method on NIH3T3 Murine Fibroblasts
[0057]NIH3T3 cells (ATCC, LGC Standards) cultured in endotoxi-free DMEM medium (Sigma-Aldrich) supplemented with 10% FCS, 100 μg / ml penicillin and 100 IU / ml streptomycin (Sigma-Aldrich) were grown in flasks until they were 80% confluent, and then harvested. Harvesting took place by incubating cells in 5 ml of PBS with 0.05% EDTA for 2 minutes, and final detachment was achieved by incubation in 0.1% trypsin and 0.05% EDTA for another 5 minutes. Cells were centrifuged at 400×G for 5 minutes. Then a total of 4.8 ml of a solution at 4° C. containing 80,000 NIH3T3 cells / 50 μl of freezing 80% DMEM, 10% FCS, 10% DMSO (AppliChem) medium was prepared (cell density determined by haernocytometer), forming the so-called freezing solution. Besides, 100 μl of complete culture medium was disposed in each cell of a 96-well plate and frozen down to −80° C. Once frozen, the plate is recovered and 50 μl / well of the said freezing soluti...
example 3
Cytotoxic Test
[0060]Balb3T3 (ATCC, LCC Standards) fibroblasts and smooth muscle aortic cells (SMAC, freshly isolated from Sprague Dawley rats, Harlan Laboratories) primary cells were cultured in complete culture medium consisting of endotoxin-free DMEM (Sigma-Aldrich) supplemented with 10% FCS, 100 μ / ml penicillin and 100 IU / ml streptomycin (Sigma-Aldrich). SMAC cells were isolated from a single male rat aorta: the aorta was carefully dissected from its origin at the left ventricle to the iliac bifurcation and cells were isolated by enzymatic digestion of the tissue with collagenase type II (Worthington). The resulting cells were allowed to grow for twelve days prior to cryopresentation. Both cell types were grown in flasks until they were 80% confluent and then harvested. Harvesting took place by incubating cells in 5 ml of PBS with 0.05% EDTA for 2 minutes, and final detachment was achieved by incubation in 0.1% trypsin and 0.05% EDTA for another 5 minutes. Cells were centrifuged ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com