Formulations having an alpha v beta 3 antagonist and an alpha 2 beta 1 antagonist for Anti-angionic therapy
a technology of beta 3 and antagonist, which is applied in the direction of angiogenin, peptide/protein ingredients, drug compositions, etc., can solve the problem of limiting this process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Human Model was Prepared to Correlate Angiogenesis With the Extracellular Matrix
[0081]In this study, we investigated the correlation between sprout angiogenesis and the integrity of an extracellular matrix (ECM) environment using in vivo and in vitro angiogenesis models. We used an αvβ3 antagonist and an α2β1 antagonist in the model, where the αvβ3 antagonist was the disintegrin echistatin, and the α2β1 antagonist was the disintegrin VP1 2 (ECL12).
Materials
[0082]A 3-D ECM model was prepared. Gelatin-coated, microcarrier beads (Cytodex-3) were purchased from Pharmacia (Uppsala, Sweden). Sterile, native bovine dermal collagen containing 95% type I collagen and 5% type III collagen (Vitrogen) was obtained from Collagen Biomaterials (Palo Alto, Calif.). Dimethyl dichlorosilane, aprotinin, dibutyryl cyclic AMP, hydrocortisone, trypsin, soybean trypsin inhibitor, and EDTA were obtained from Sigma Chemical Co. (St. Louis, Mo.). Endothelial cell basal medium (EBM), endothelial cell growth...
example 2
Cell Migration and Capillary Sprout Formation was Identified in Fibrin Gels and Type I Collagen Gels in the Human Model
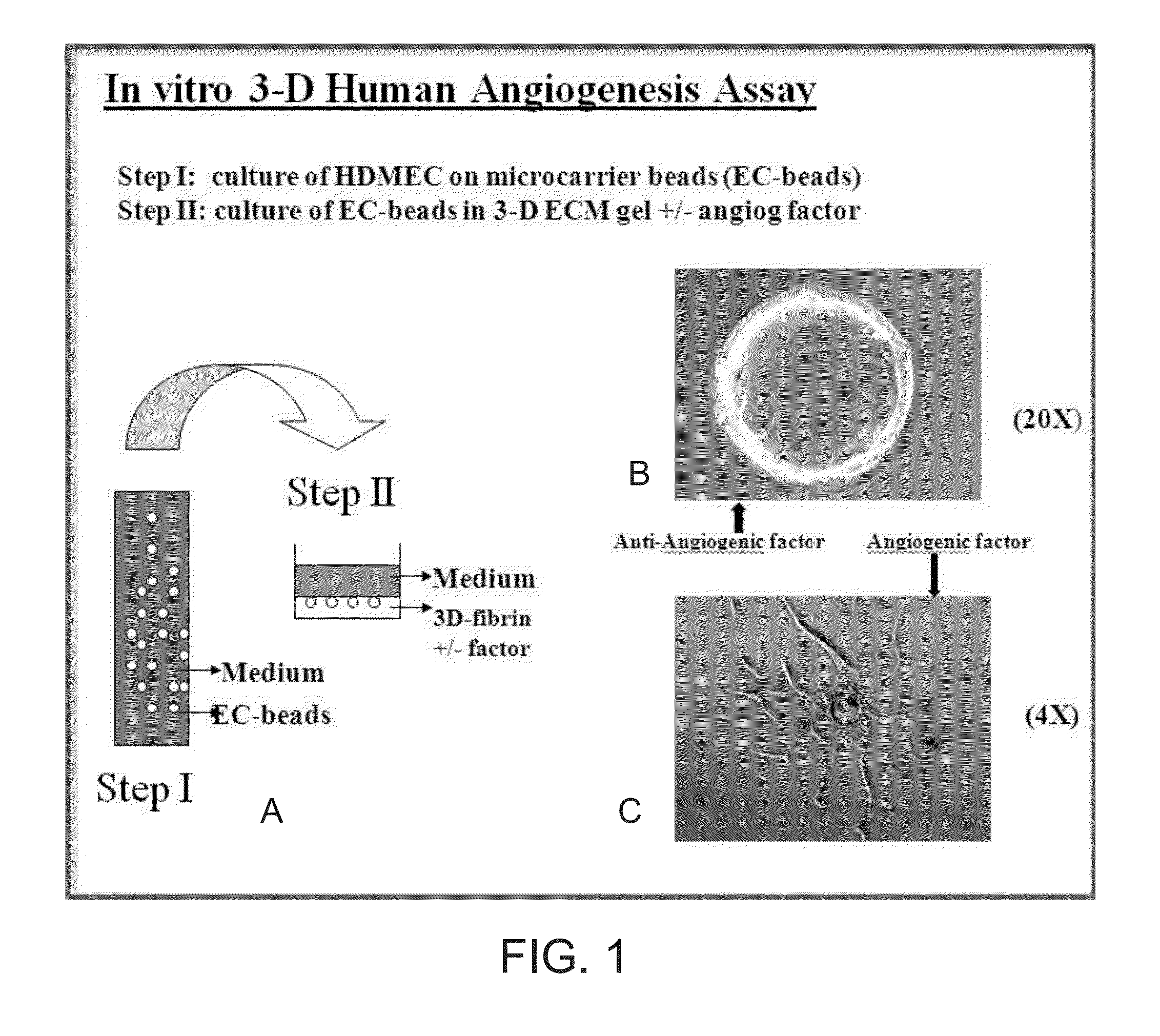
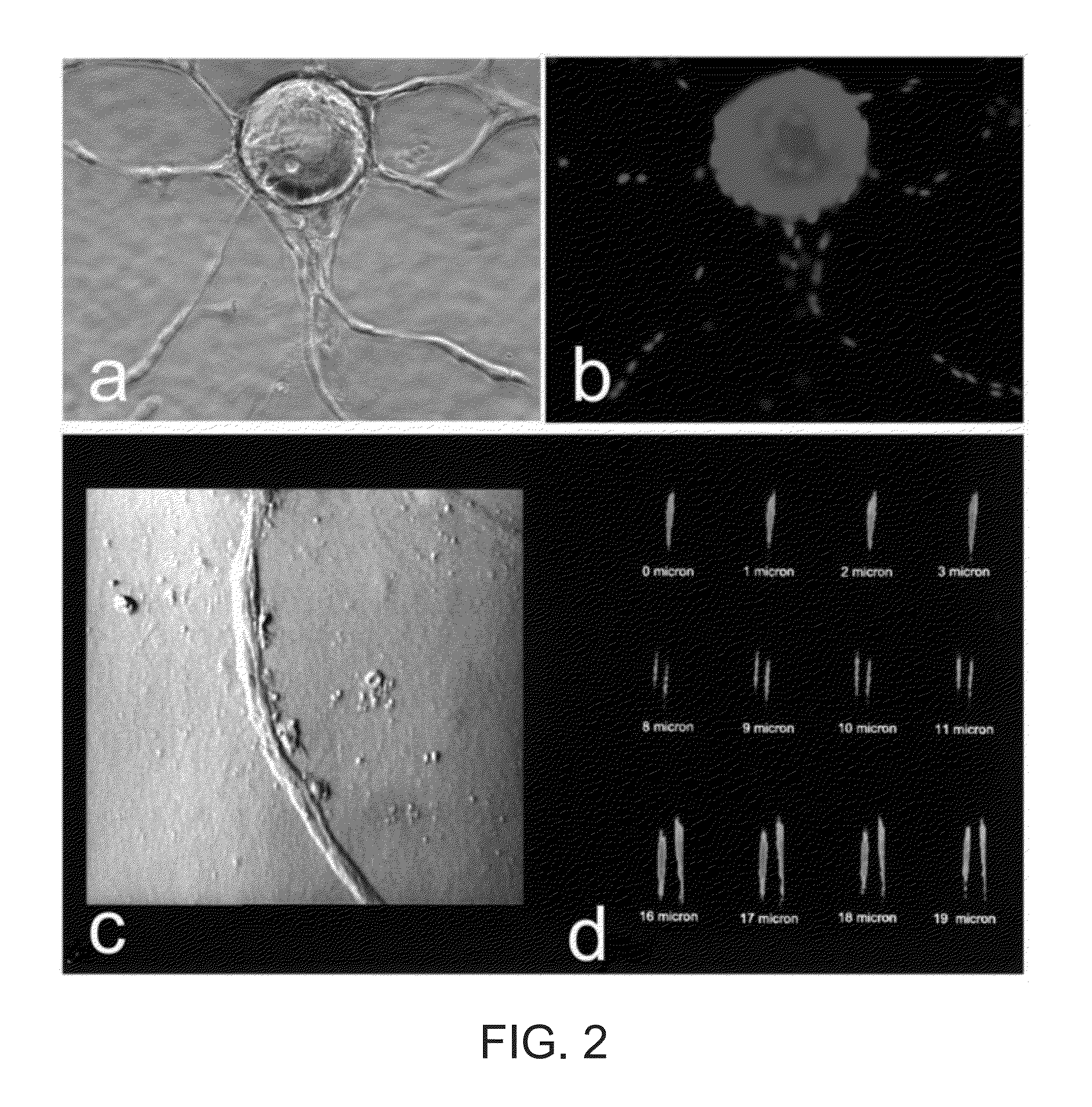
[0085]FIGS. 1A-1C illustrate a study of human microvascular endothelial cell angiogenesis, according to some embodiments. A microcarrier, in vitro angiogenesis assay, previously designed to investigate bovine pulmonary artery endothelial cell angiogenic behavior in bovine fibrin gels, was modified for the study of human microvascular endothelial cell angiogenesis. The HDMEC were isolated from human neonatal foreskins and used, as described above, and images were captured at various magnifications, where the effect of angiogenic factors on sprout angiogenesis was quantified visually by counting the number and percent of EC-beads with capillary sprouts.
[0086]FIG. 1A shows the process in Step I, where human fibrinogen, isolated as previously described, was dissolved in M199 medium at a concentration of 1 mg / ml (pH 7.4) and sterilized by filtering through a 0.22 micron ...
example 3
Integrin Receptors Were Identified Using Porcine Cutaneous Wounds and Immunofluorescence Staining
[0091]Porcine cutaneous wounds were harvested at various times and then immunoprobed for expression of integrin receptors. See Xu, J and Clark, R. The Journal of Cell Biology 132:239-249(1996). Briefly, full-thickness wounds were made with an 8-mm punch on the backs of White Yorkshire pigs and harvested at the times indicated. Specimens were bisected; one half was fixed in formalin and stained with MASSON trichrome, the other half was frozen in liquid nitrogen for immunofluorescence studies. Anti-laminin antibodies (Gibco BRL) conjugated with biotin were used to identify wound vasculature. All antibodies were used at dilutions that gave maximal specific fluorescence and minimal background fluorescence on frozen tissue specimens. Bound antibody was detected by the avidin-biotin-complex (ABC) technique. Stained specimens were observed and photographed using a NIKON Microphot FXA epifluores...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com