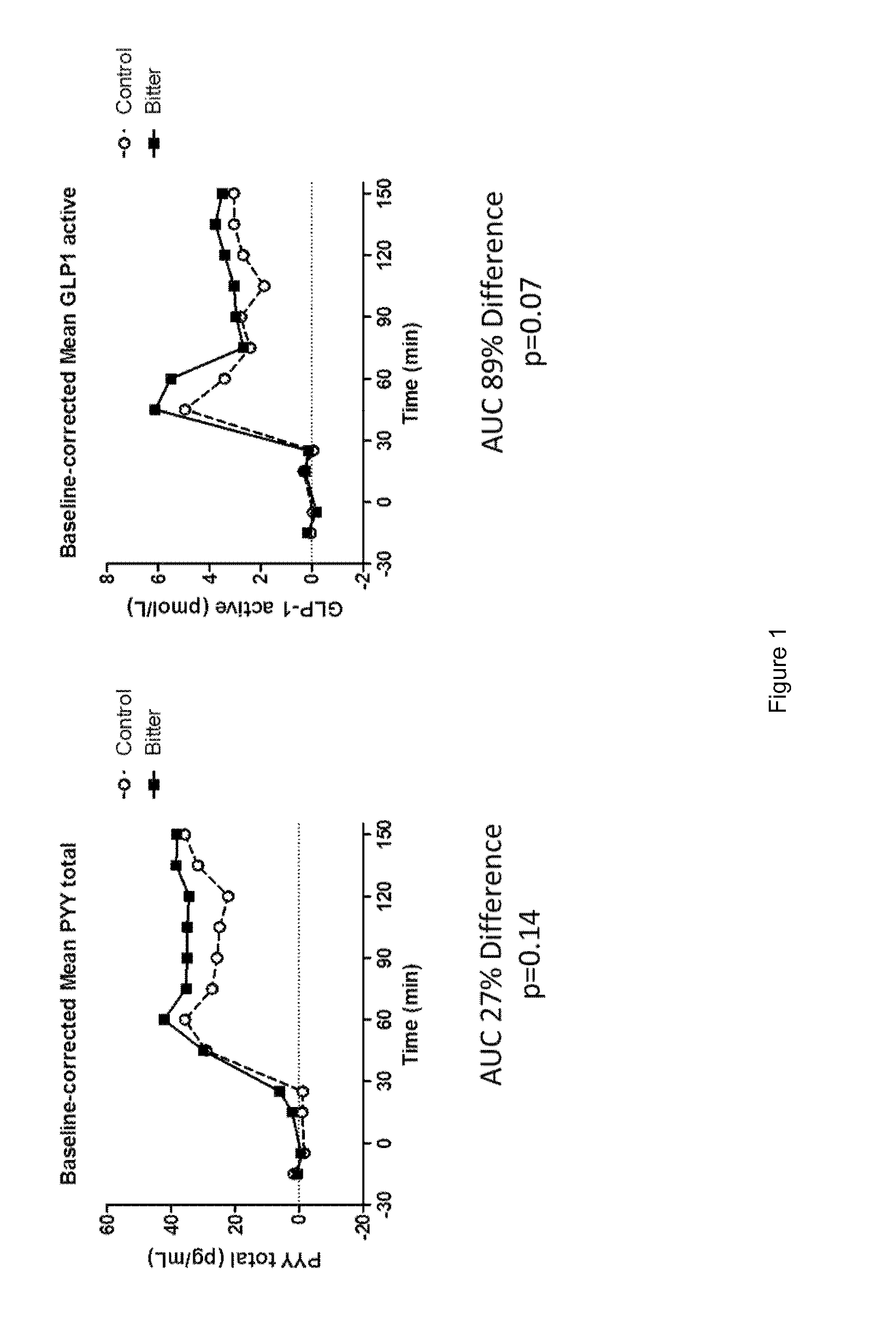
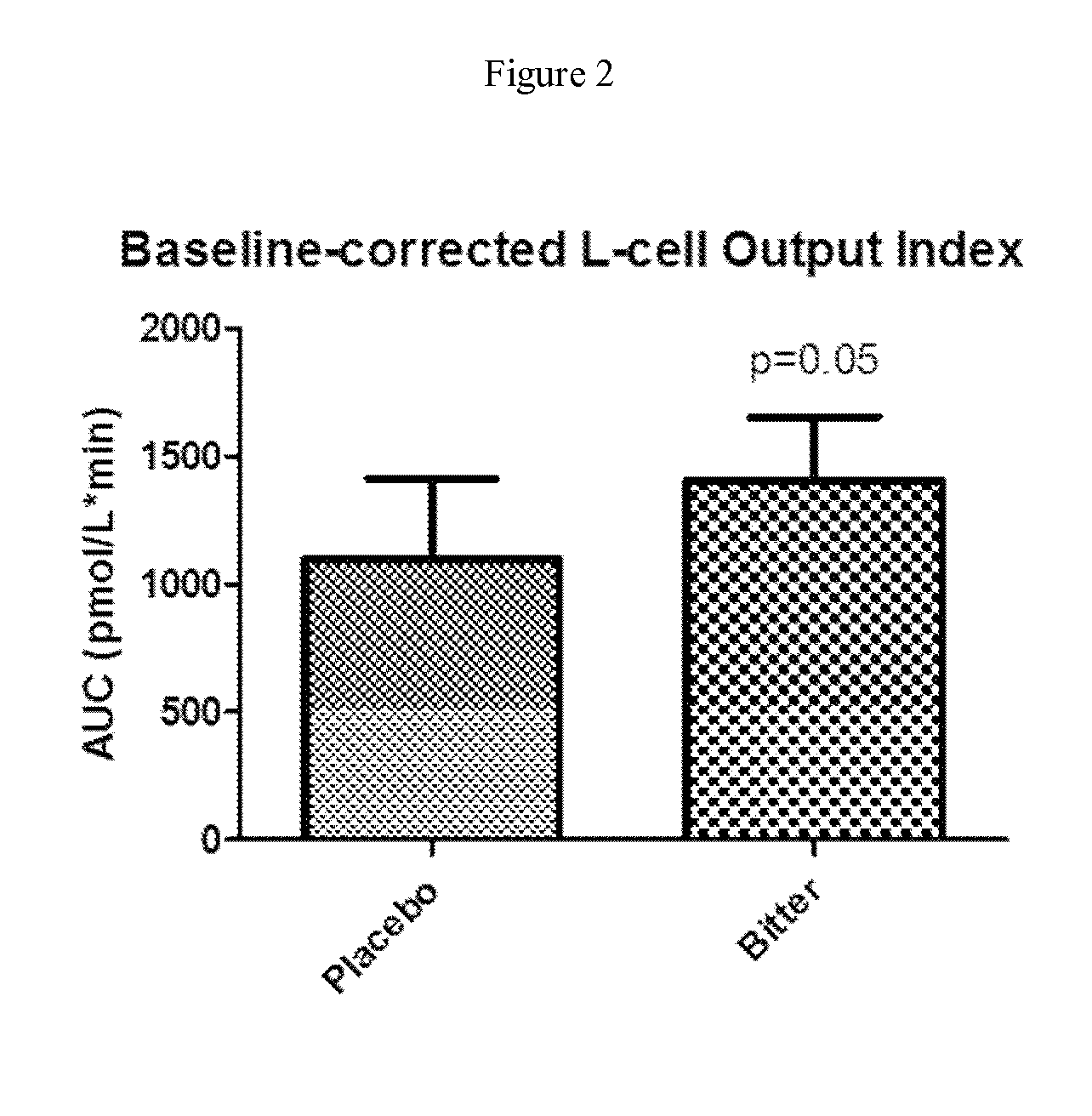
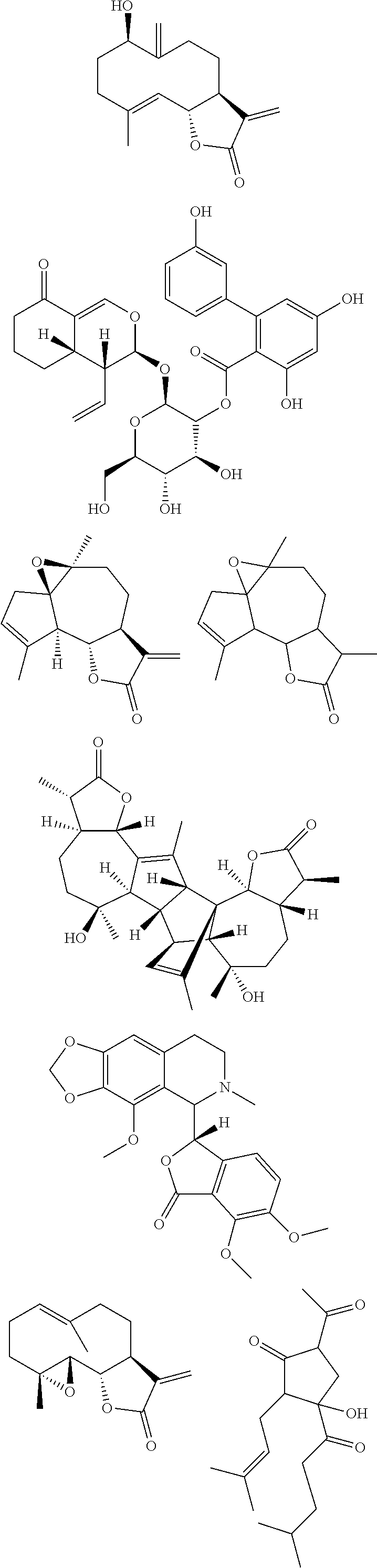
Chemosensory Receptor Ligand-Based Therapies
a chemosensory receptor and ligand technology, applied in the field of chemosensory receptor ligand-based therapies, can solve the problems of shortened lifespan, reduced work productivity, and lowered quality of life, and achieve the effects of reducing food intake, healthy weight, and impaired fasting glycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Example 1a
Upper GI Administration of One Chemosensory Receptor Ligand in Diabetic Rats
[1370]Numerous established and accepted diabetic rat models exist for the assessment of therapies for the treatment of diabetes. A single chemosensory receptor ligand (e.g., sweet) can be assayed for the treatment of diabetes in this established diabetic rat model as detailed in the example below.
[1371]Diabetic rats and Wistar rats are selected for administration of the chemosensory receptor ligand (e.g., sucralose) for the treatment of diabetes. Animals are grouped according to dosage, and increasing dosages (range of 0.01-100 mg / kg) are utilized. Chemosensory receptor ligands are instilled into the animals via silastic tubing inserted into the duodenum through the mouths of the lightly anesthetized animals.
[1372]Optionally, Dipeptidyl Peptidase IV (DPP-IV) is inhibited in designated groups, or all, of the test animals to prevent degradation of the target hormones by endogenous peptidases. DPP-IV ...
example 1b
[1380]Alternatively, the chemosensory receptor ligand, if not metabolized, is administered with a cognate metabolite in the experimental protocol above. For example in an alternative protocol, sucralose is administered along with glucose. The ligand may be administered in increasing doses with respect to a fixed dose of the cognate metabolite and vice versa.
example 1c
[1381]Alternatively, the experimental protocol above is performed with industry standard Diet Induced Obese rats and applicable controls (healthy rats). Parameters unique to the obesity systems are modified based on known standard assay conditions. Samples are collected and hormone assays performed as described above. Additional hormones, such as glycentin and uroguanylin may be measured.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com