Pharmaceutical composition
a technology of pharmaceutical compositions and compositions, applied in the field of pharmaceutical compositions, can solve the problems of long time-consuming heating and shaking of the process, affecting the stability of the produced drug formulation, and problematically impairing operability, so as to enhance the solubility and stability of the injection formulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0044]Injection solutions for drip infusions, which are the pharmaceutical composition of the present invention, were prepared according to the below-described formulations. Specifically, the formulated ingredients were stirred at 30° C. for dissolution. In each case, the time required for dissolution was measured. The results are shown in Table 2. As is clear from Table 2, the pharmaceutical composition of the present invention has been found to provide enhanced solubility of 1-(1-hydroxymethyl-2,3-dihydroxypropyl)oxymethyl-2-nitroimidazole (compound 1). Note that a sample of Referential Example 1 contained no creatinine.
TABLE 1IngredientCompound (1)6 gCreatinineamount shown in Table 2Waterto total volume of 100 mL
TABLE 2Mass ofTime required forSamplescreatinine (g)dissolution (min)Pharmaceutical0.0525compn. 1Pharmaceutical0.125compn. 2Pharmaceutical0.220compn. 3Ref. Ex. 1030
[0045]When these samples were allowed to stand at 20° C., no change was observed within five hours from star...
example 2
[0046]In a manner similar to Example 1, injection solutions for drip infusion (i.e., pharmaceutical compositions 4 to 6), which are pharmaceutical compositions of the present invention, were prepared according to the below-described formulations.
TABLE 3IngredientCompound (1)5 gCreatinineamount shown in Table 4Waterto total volume of 100 mL
TABLE 4Mass ofSamplescreatinine (g)Pharmaceutical0.02compn. 4Pharmaceutical0.1compn. 5Pharmaceutical0.2compn. 6Ref. Ex. 20
example 3
Test Example 1
[0047]Each of the above-produced pharmaceutical compositions
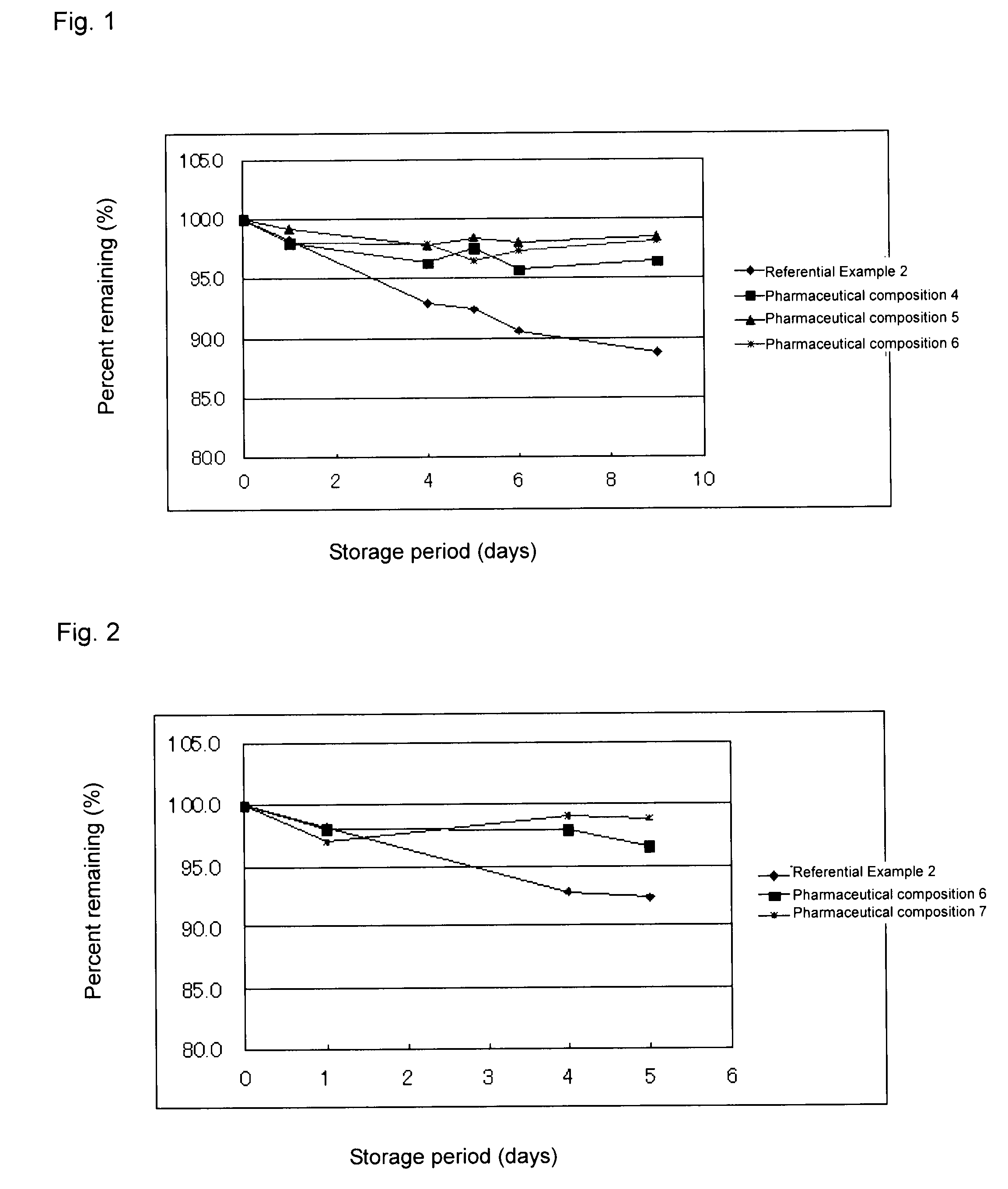
[0048]4 to 6 and the composition of Reference Example 2 was stored under severe conditions (55° C.) for 9 days, and the compound (1) content of the composition was determined through HPLC. The determined value was divided by 5, followed by multiplication by 100, to thereby obtain percent content (%). The results are shown in FIG. 1. As shown in FIG. 1, the pharmaceutical compositions of the present invention exhibited excellent stability. HPLC was carried out under the following conditions: ODS column (4.6 mm×250 mm), column temperature (40° C.), flow rate 1 mL / min, mobile phase (1% aqueous methanol), and detection UV region (320 nm).
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass % | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com