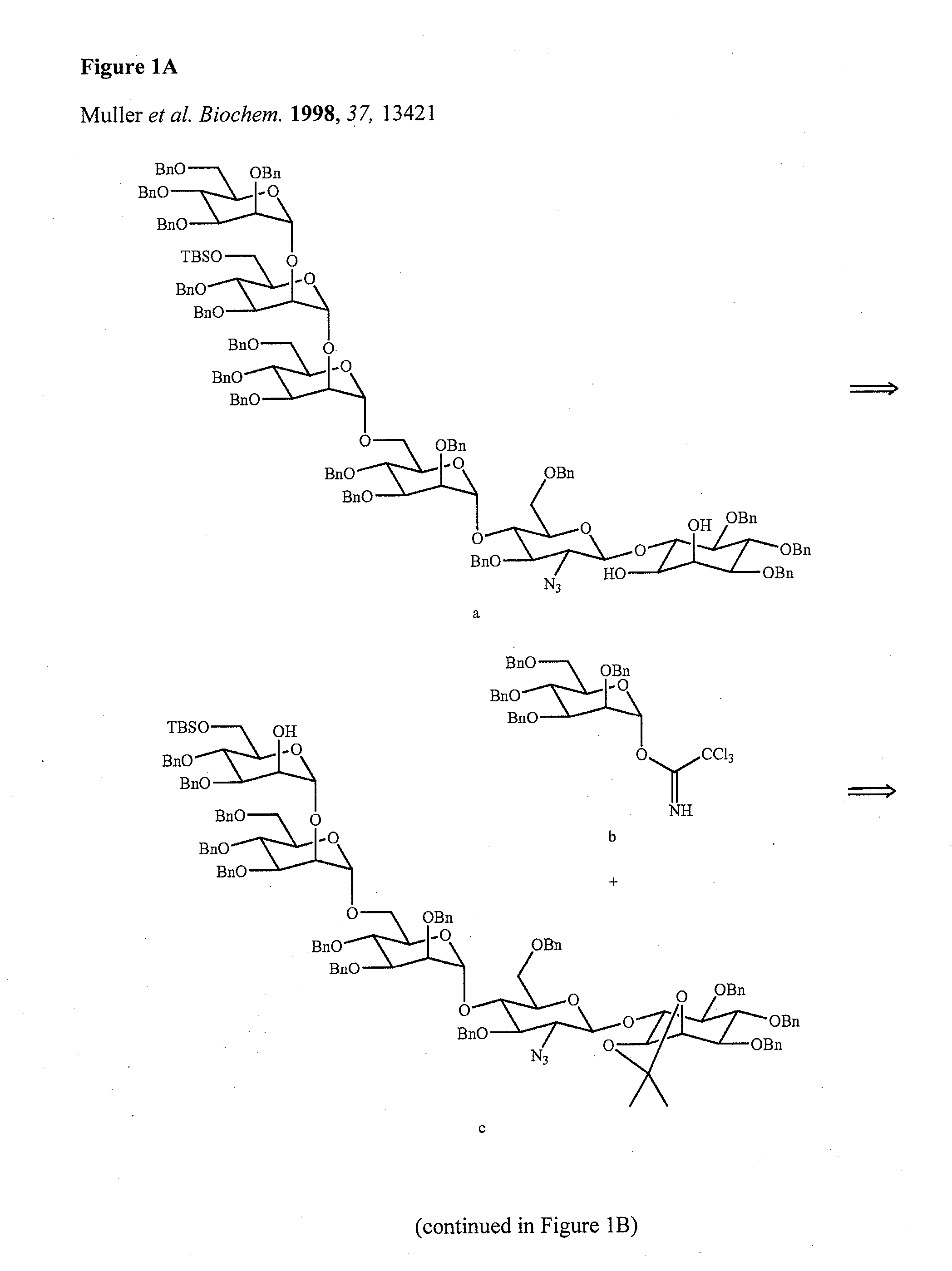
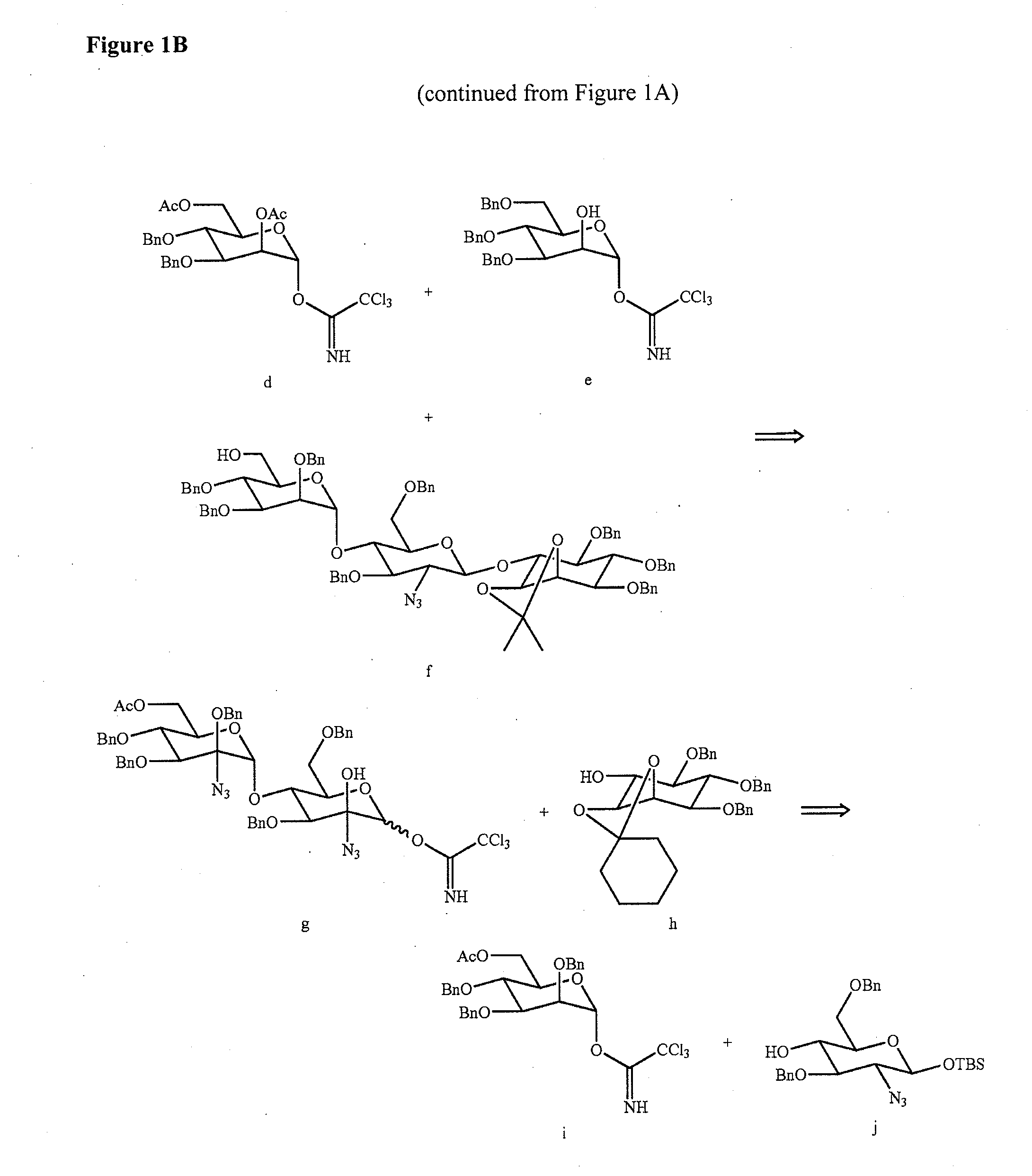
Solid-Phase and Solution-Phase Synthesis of Glycosylphosphatidylinositol Glycans
a glycosylphosphatidylinositol and solution-phase technology, which is applied in the direction of antibody medical ingredients, carrier-bound antigen/hapten ingredients, immunological disorders, etc., can solve the problem of no solid-phase or automated synthesis reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0151]
[0152]Ethyl-4-O-acetyl-2-azido-3,6-di-O-benzyl-2-deoxy-thio-β-D-glucopyranoside 5. [α]24D: −43.5° (c 1.07, CH2Cl2); IR (thin film) 2916, 2108, 1743, 1222, 1047 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.40-7.29 (m, 10H), 5.06-5.02 (m, 1H), 4.86 (d, J=11.3 Hz, 1H), 4.68 (d, J=11.3 Hz, 1H), 4.52 (s, 2H), 4.34 (d, J=9.5 Hz, 1H), 3.59-3.48 (m, 6H), 2.83-2.73 (m, 2H), 1.87 (s, 3H), 1.35 (t, J=7.3 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 169.9, 138.0, 137.8, 128.8, 128.6, 128.3, 128.1, 128.0, 84.5, 82.8, 77.9, 75.9, 73.8, 71.1, 69.9, 66.0, 25.0, 21.0, 15.4; FAB MS m / z (M+Na)+ calcd 494.1726, found 494.1716.
example 2
[0153]
[0154]6-O-Acetyl-2,3,4-tri-O-benzyl-α-D-mannopyranose trichloroacetimidate 6. [α]24D: +34.4° (c 2.18, CH2Cl2); IR (thin film) 2938, 2880, 1707, 1683, 1220 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.53 (s, 1H), 7.38-7.21 (m, 14H), 6.29 (d, J=2.1 Hz, 1H), 4.91 (d, J=10.7 Hz, 1H), 4.73 (s, 2H), 4.61-4.55 (m, 3H), 4.33 (dd, J=2.1, 12.2 Hz, 1H), 4.24 (dd, J=4.3, 11.9 Hz, 1H), 4.00-3.90 (m, 4H), 3.84-3.83 (m, 1H), 2.00 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 170.8, 160.3, 137.8, 137.8, 137.7, 128.5, 128.4, 128.3, 128.3, 128.0, 127.8, 127.8, 95.6, 78.8, 75.3, 73.6, 73.2, 72.7, 72.5, 72.2, 63.0, 20.8; FAB MS m / z (M+Na)+ calcd 658.1137, found 658.1123.
example 3
[0155]
[0156]2-O-Acetyl-3,4-di-O-benzyl-6-O-triisopropylsilyl-α-D-mannopyranose trichloroacetimidate 8. [α]24D: +43.4° (c 2.20, CH2Cl2); IR (thin film) 2940, 2865, 1752, 1674, 1229 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.63 (s, 1H), 7.38-7.26 (m, 10H), 6.26 (d, J=1.8 Hz, 1H), 5.46 (dd, J=2.1, 3.1 Hz, 1H), 4.93 (d, J=10.4 Hz, 1H), 4.75 (d, J=11.3 Hz, 1H), 4.71 (d, J=10.7 Hz, 1H), 4.62-4.57 (m, 1H), 4.17 (t, J=9.8 Hz, 1H), 4.09-4.05 (m, 2H), 3.95 (d, J=11.3 Hz, 1H), 3.85 (dd, J=1.5, 9.8 Hz, 1H), 2.16 (s, 3H), 1.15-1.05 (m, 22H); 13C-NMR (125 MHz, CDCl3) δ 170.8, 160.7, 139.1, 138.3, 129.1, 129.1, 129.0, 128.8, 128.6, 128.5, 96.2, 91.6, 77.9, 76.3, 74.0, 72.8, 68.1, 62.8, 21.6, 18.7, 18.6, 12.8; FAB MS m / z (M+Na)+ calcd 724.2007, found 724.2006.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com