Compositions and methods for detecting cancer
a technology of sialylated glycans and detection methods, applied in the field of sialylated glycans and antibodies, can solve the problems of large variability in immune responses, lack of sensitivity insufficient specificity and speciality for early detection, so as to facilitate tumor progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0092]Serum samples. A total of 386 cancer cases and control human sera were studied, with approval from the Institutional Review Board of the University of California, San Diego. Written, informed consent was obtained in advance. Sera were collected from patients seen at the Moore's UCSD Cancer Center Clinic who did not have known or suspected pregnancy or infection. De-identified serum was placed into 12 separate bar-coded aliquots and stored at −80° C. EDRN common data elements (CDE) related to sample handling were captured prospectively and recorded in the Biorepository database. We tested sera from 175 breast cancer patients and other types of carcinomas including prostate (39), ovary (29), lung (14), colon (22), pancreas (16), endometrium (11), as well as controls (80) matched for gender and, as possible, for age. Control sera were obtained from patients seen at the Cancer Center clinics who do not have a diagnosed cancer, including those with benign tumor...
example 2
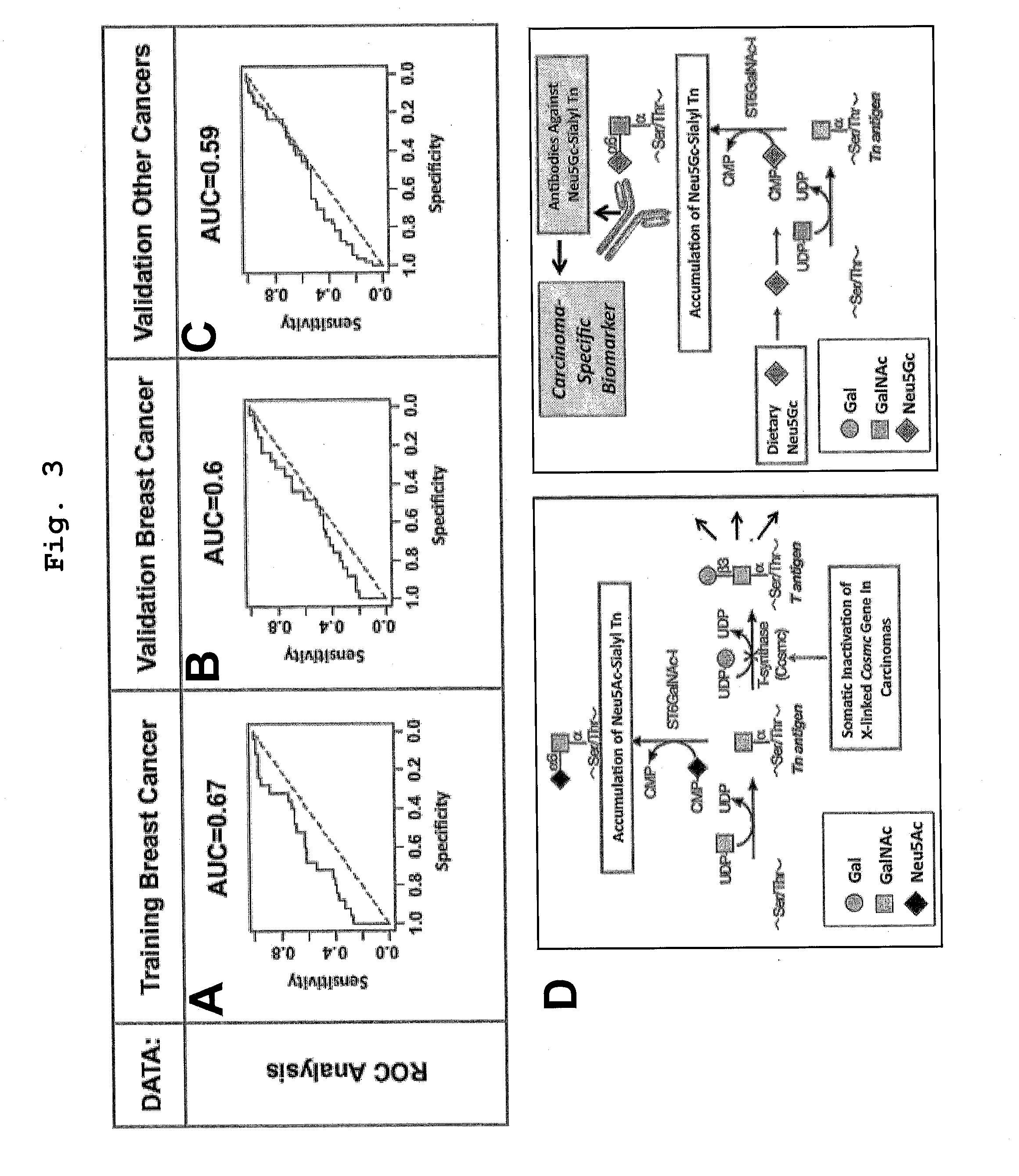
[0108]In an exemplary embodiment, we used a unique sialoglycan-microarray to describe antibodies against a diet-related antigen as novel type of human serum carcinoma-biomarker. This establishes the new concept that a diet-derived antigen can metabolically-incorporate into tumors, generating a novel antigen detected by the immune system.
TABLE 1Review of the studied subjects, by cancer type.Number of subjectsexcluding metastaticCase / ControlNumber of subjectscancersType ofBreast175141cancerProstate3934Ovary2926Lung146Colon2215Pancreatic167Endometrial1111Total cases306240Controls8080Total Cases + Controls386320
TABLE 2List of Glycans Studied on the Array.O-Acety-GlycanlationGlycanTypeStatusNo.CompoundAc9OAc1Neu5,9Ac2α2-3Galβ1-4GlcNAcβProNH2Gc9OAc2Neu5Gc9Acα2-3Galβ1-4GlcNAcβProNH2Ac9OAc3Neu5,9Ac2α2-6Galβ1-4GlcNAcβProNH2Gc9OAc4Neu5Gc9Acα2-6Galβ1-4GlcNAcβProNH2Ac—5Neu5Acα2-6GalNAcαProNH2Gc—6Neu5Gcα2-6GalNAcαProNH2Ac9OAc7Neu5,9Ac2α2-3-Galβ1-3GlcNAcβProNH2Gc9OAc8Neu5Gc9Acα2-3Galβ1-3GlcNAcβPr...
example 3
[0111]Results: Evaluation and Optimization of Sialoglycan-Microarray for Biomarker Discovery: Array Sensitivity Analysis and Validation.
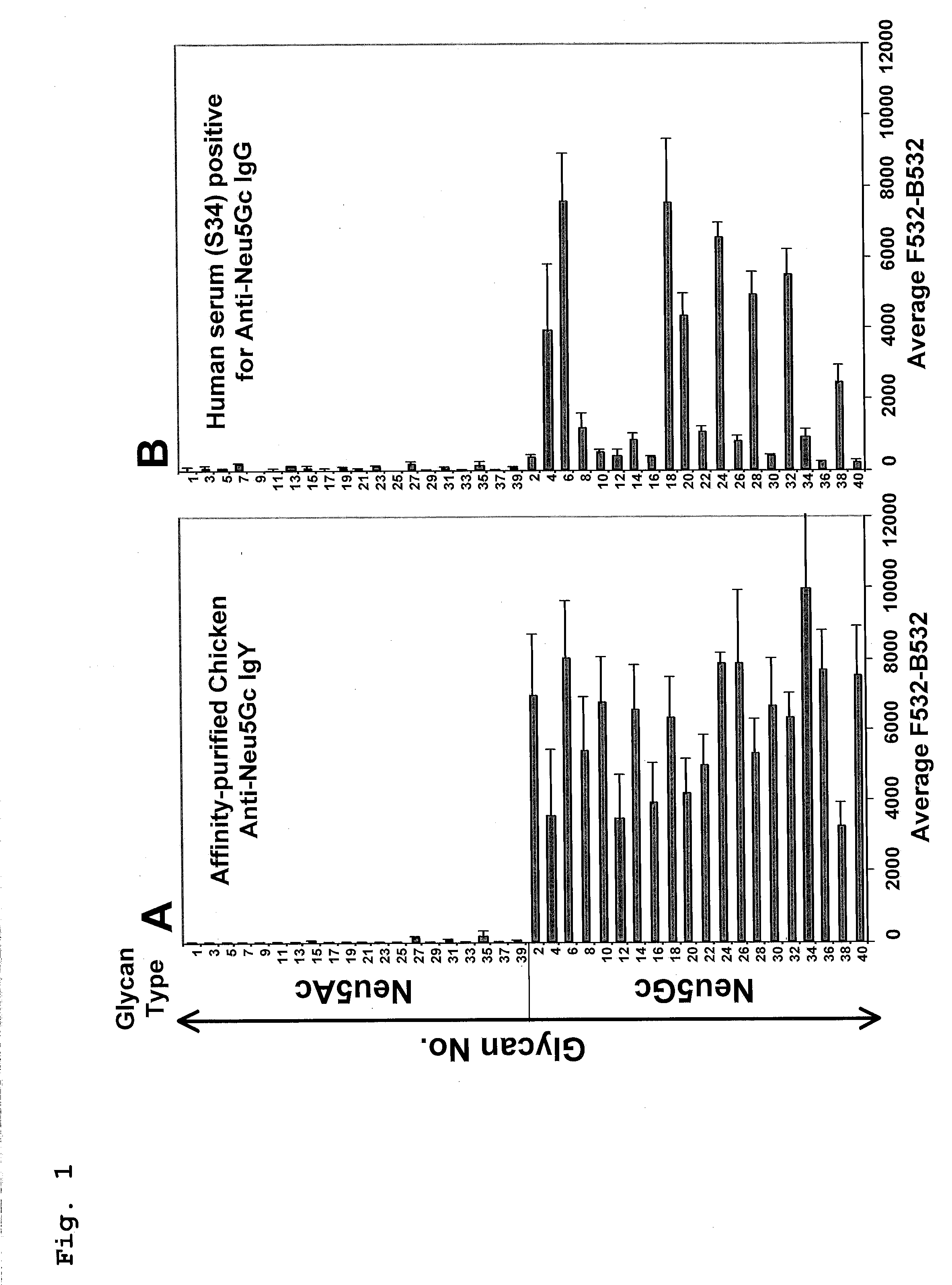
[0112]A microarray approach permits high-throughput analysis of multiple samples and is valuable for comparative human serum profiling (33). To screen multiple anti-Neu5Gc IgGs in human sera, we used a highly efficient chemoenzymatic approach (27-29) to synthesize 40 sialylated glycans representing potentially common sialyloglycans on tumor cells. These 20 matched sialoglycan-pairs terminated with Neu5Gc or Neu5Ac, (Table 2; differing by one oxygen atom) and some of their 9-O-acetylated forms, were printed on Epoxy-coated slides in a range of concentrations. Slide print quality was monitored with polyclonal affinity-purified chicken anti-Neu5Gc IgY (34), and with a positive control human serum, both showing specific high reactivity to multiple Neu5Gc-glycans but not Neu5Ac-glycans. Next, sera from cancer or non-cancer patients were tested on the sia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Acceleration | aaaaa | aaaaa |
| Acceleration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com