Treatment Method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
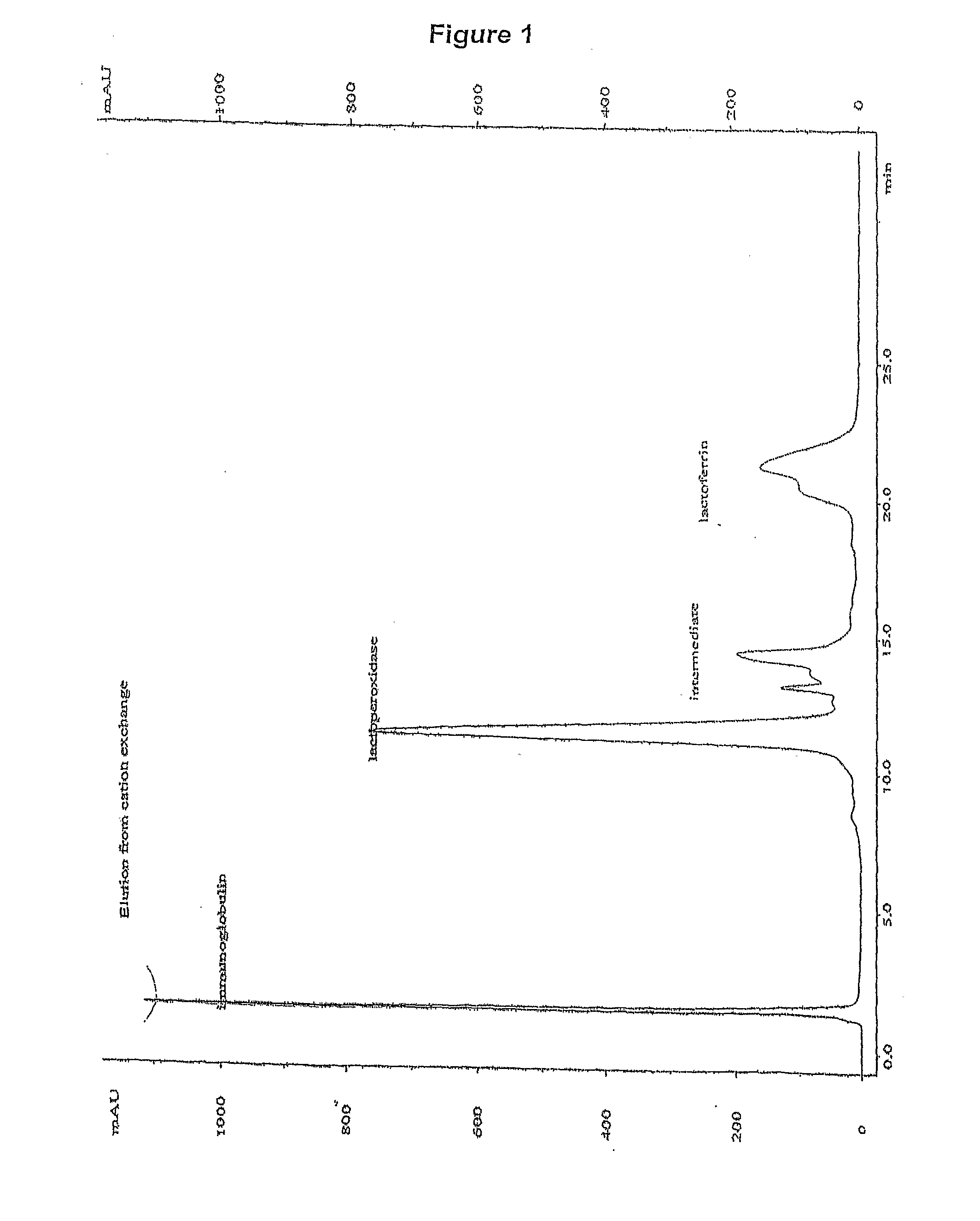
[0227]FIG. 1 shows the elution profile of the cationic fraction from cation exchange. This represents all the protein peaks (as detected at 280 nm) that would be present in a single fraction eluted in a gradient from 80-100 mS. The main components in the cationic fraction are immunoglogulin, lactoperoxidase, lactoferrin, and a group of minor components that include angiogenin.
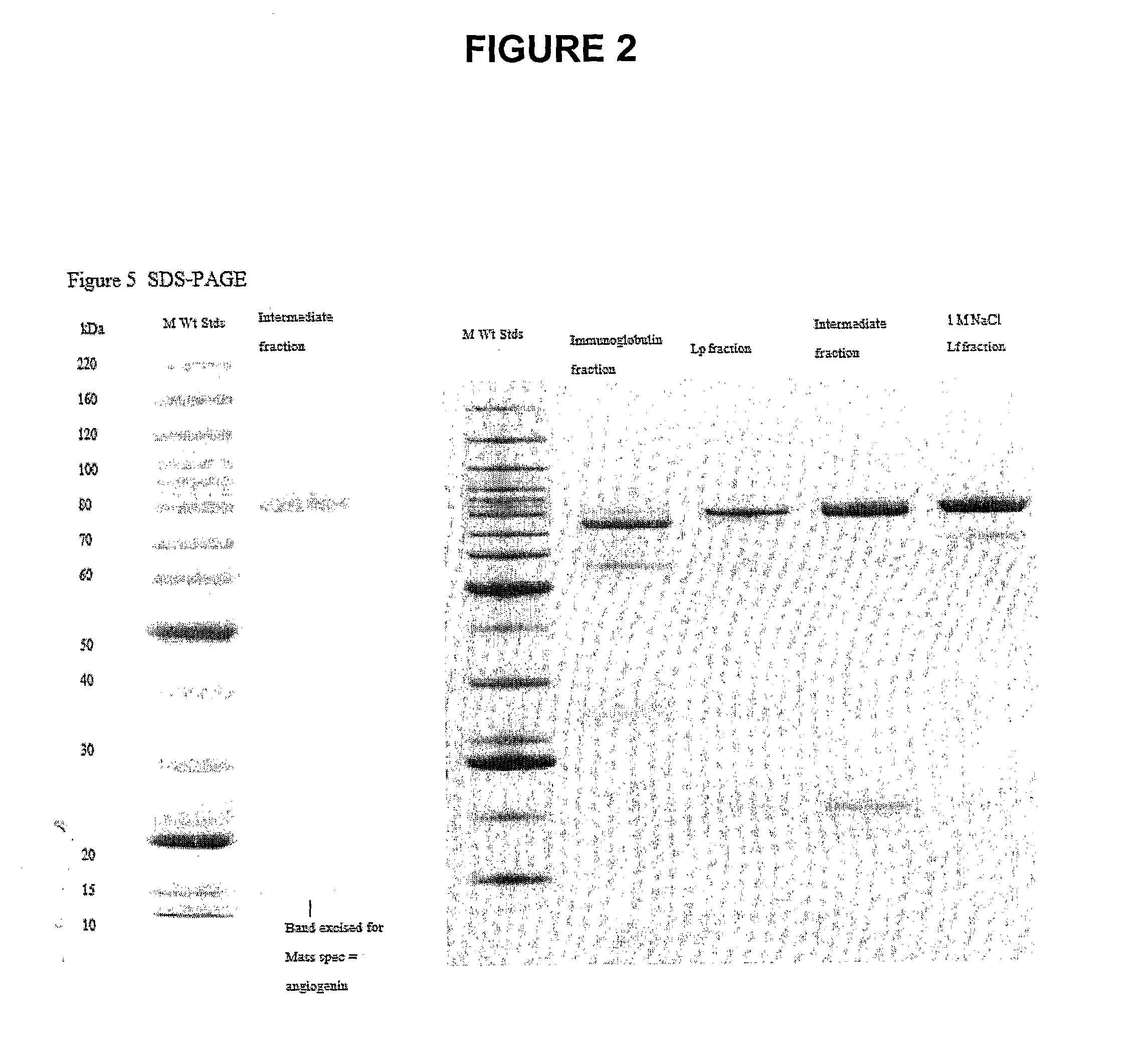
[0228]FIG. 2 shows the main fractions separated on SDS-PAGE, and indicates the band that was excised for Mass Spectroscopy and identified as bovine angiogenin.
[0229]The immunoglobulin fraction shows PIGR (76 kDa) as the predominant band, and the heavy (52 kDa) and light chains of immunoglobulin.
[0230]The Lp fraction is mainly lactoperoxidase with a small amounts of heavy and light chains of immunoglobulin and angiogenin.
[0231]The intermediate fraction has a prominent band of lactoperoxidase and lactoferrin (80 kDa) and a band at around 15 kDa that was identified by Mass Spectrometry as angiogenin, a band at app...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com