Method for treating surface of oxide glass with fluorinating agent
a fluorinating agent and oxide glass technology, applied in the direction of instruments, optical elements, coatings, etc., can solve the problems of poor adhesion of fluorine to the oxide glass, the use of expensive materials, and the limited amount of material which can be vaporized, etc., to achieve excellent adhesion without deterioration of surface properties, impart desired properties, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
of the Present Invention
[0060]In the first embodiment of the present invention, the concentration of hydrogen fluoride at the surface of oxide glass with which the gaseous fluorinating agent is in contact, is controlled to be at most 1 mol % by carrying out the procedure of contacting the surface of oxide glass with the gaseous fluorinating agent in a closed container containing the gaseous fluorinating agent and a solid metal fluoride.
[0061]In the first embodiment of the present invention, the concentration of hydrogen fluoride at the surface of oxide glass with which the fluorinating agent is in contact, is controlled to be at most 1 mol %, by letting the solid metal fluoride adsorb hydrogen fluoride formed by a side reaction from the fluorinating agent by inclusion of moisture or a volatile organic compound.
[0062]The solid metal fluoride to be used is not particularly limited, and it is preferably one selected from the group consisting of alkali metal fluorides, alkaline earth me...
second embodiment
of the Present Invention
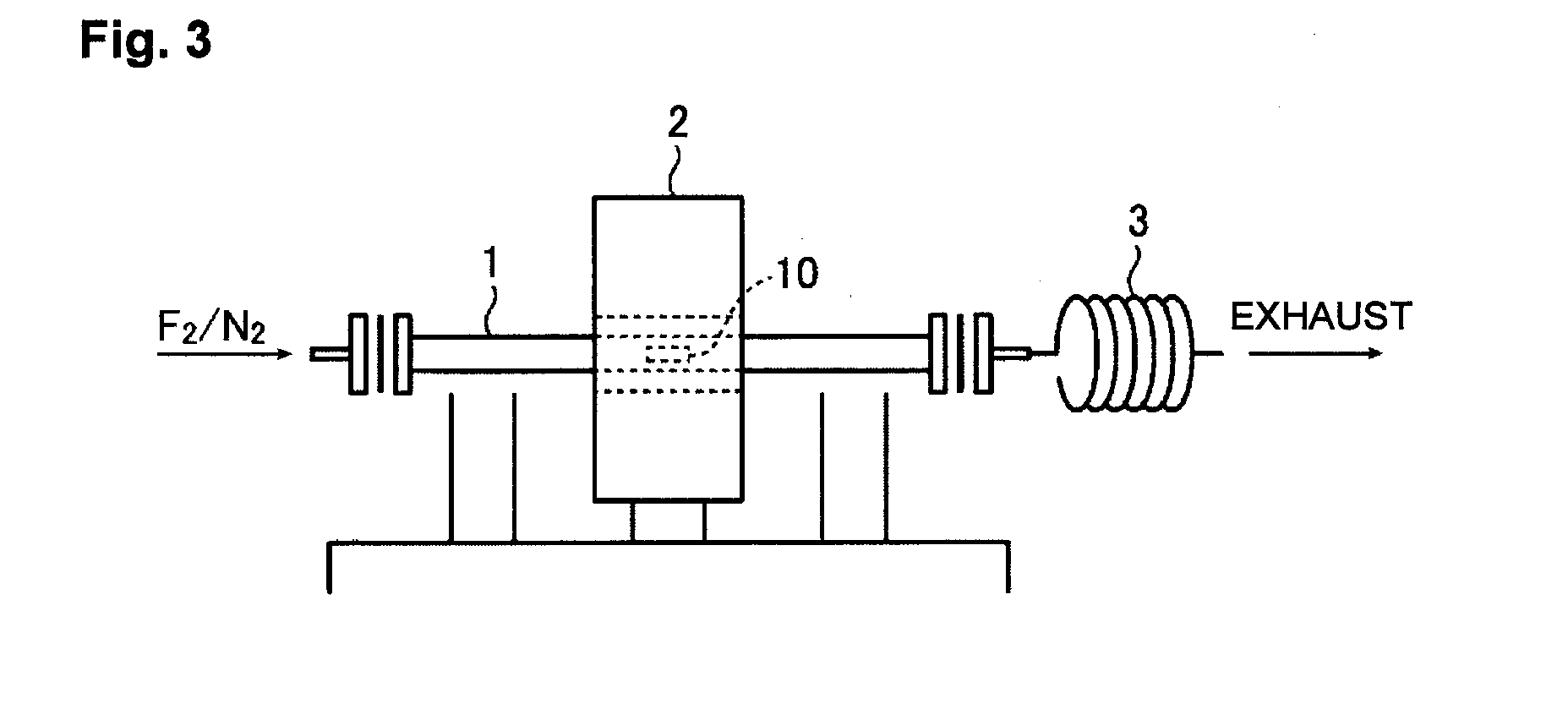
[0070]In the second embodiment of the present invention, the concentration of hydrogen fluoride at the surface of oxide glass with which the fluorinating agent is in contact, is controlled to be at most 1 mol % by carrying out a procedure of contacting the surface of oxide glass with the gaseous fluorinating agent by setting the oxide glass in a pipe line in which the gaseous fluorinating agent continuously flows.
[0071]In the second embodiment of the present invention, the concentration of hydrogen fluoride at the surface of oxide glass is controlled to be at most 1 mol % by carrying off hydrogen fluoride formed by a side reaction from the fluorinating agent by inclusion of moisture or a volatile organic compound, from the surface of the oxide glass by the gaseous fluorinating agent continuously flowing.
[0072]In the second embodiment of the present invention, a preferred range of the pressure of the gaseous fluorinating agent in the pipe line is the same as t...
example 1
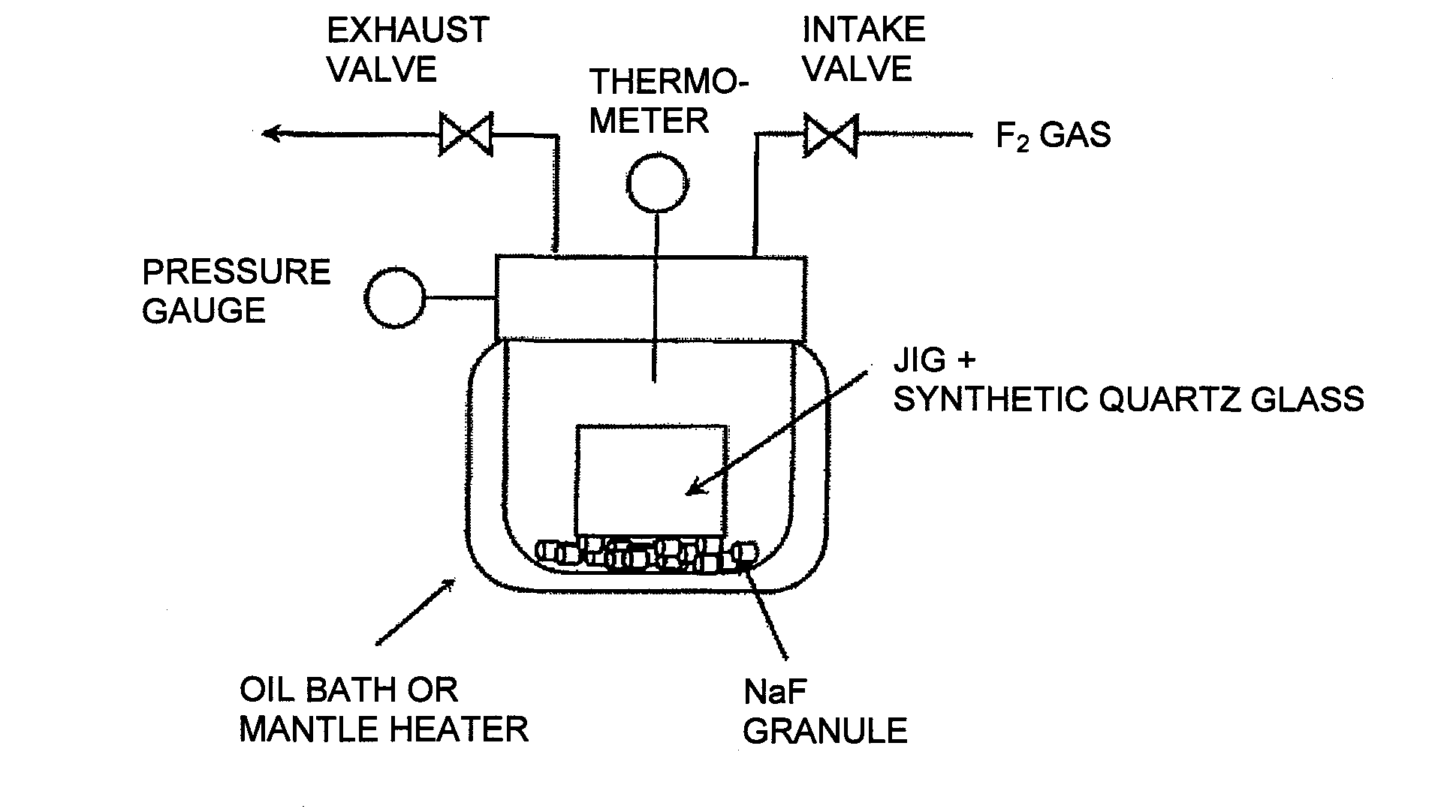
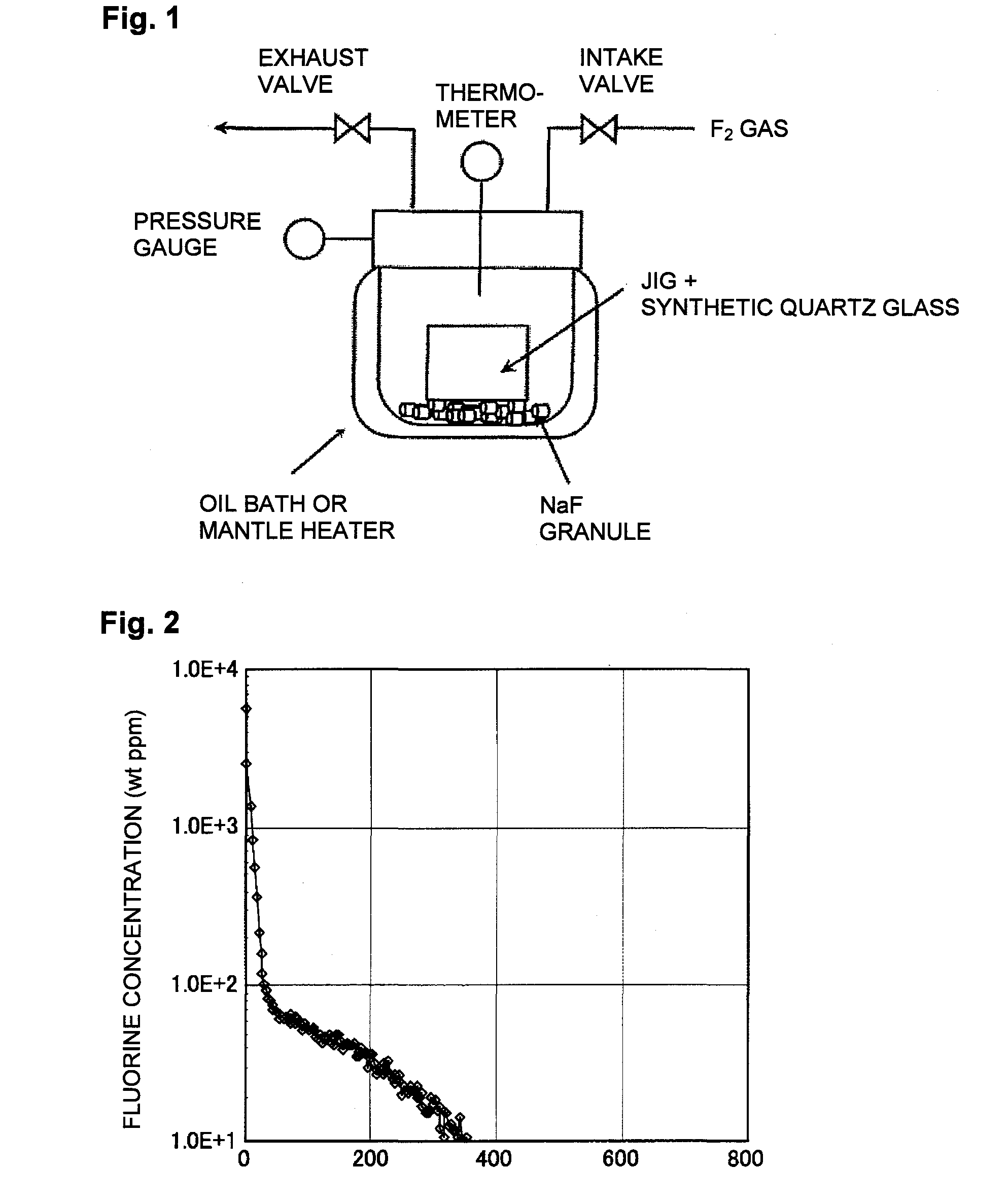
[0093]In Example 1, the first embodiment of the present invention was carried out by using the apparatus as shown in FIG. 1.
[0094]As the oxide glass, a flat plate (2 cm×2 cm×2.5 mm in thickness) of synthetic quartz glass was used. The flat plate of synthetic quartz glass was supported by a jig made of SUS 316 and put together with the jig into an autoclave made of nickel (volume: 1 L).
[0095]Then, 15 g of NaF pellets (manufactured by STELLA CHEMIFA CORPORATION) were introduced into the autoclave so that they would not contact the synthetic quartz glass plate. Then, the autoclave was heated from the exterior by using an oil bath to raise the temperature from room temperature to 80° C. at a temperature raising rate within a range of from 0.5 to 2° C. / min.
[0096]Then, while the interior of the device was maintained at 80° C., vacuum degassing was carried out until the pressure in the apparatus became not higher than 266 Pa and held for one hour. This operation was intended to remove the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| dew point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com